Home /
Expert Answers /
Chemistry /
q4-acetic-acid-and-water-react-to-form-hydronium-cation-and-acetate-anion-like-this-mathrm-hc-pa967
(Solved): Q4 Acetic acid and water react to form hydronium cation and acetate anion, like this: \[ \mathrm{HC ...
Q4
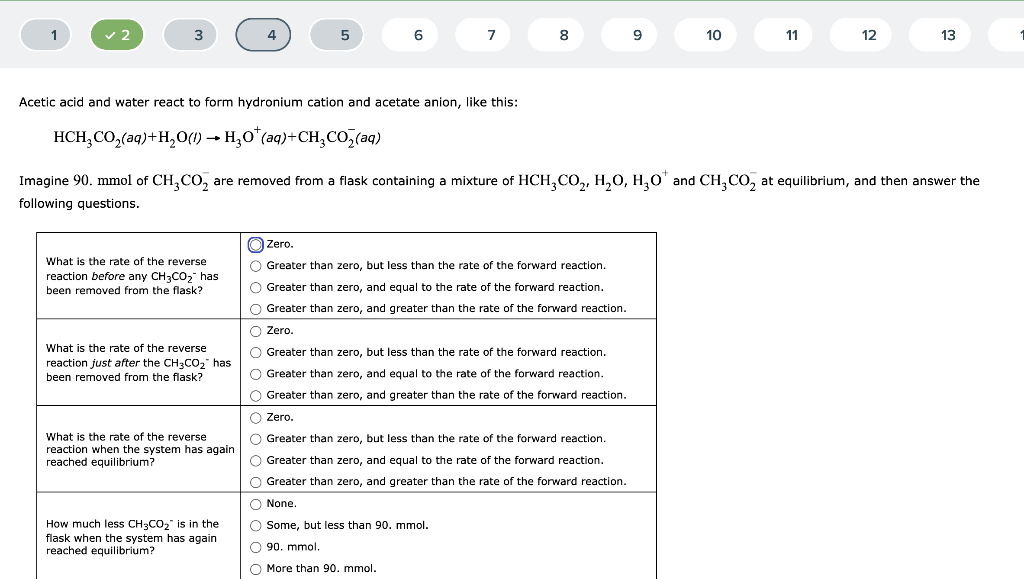
Acetic acid and water react to form hydronium cation and acetate anion, like this: \[ \mathrm{HCH}_{3} \mathrm{CO}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \rightarrow \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{CH}_{3} \mathrm{CO}_{2}^{-}(a q) \] Imagine 90. mmol of \( \mathrm{CH}_{3} \mathrm{CO}_{2}^{-} \)are removed from a flask containing a mixture of \( \mathrm{HCH}_{3} \mathrm{CO}_{2}, \mathrm{H}_{2} \mathrm{O}_{1} \mathrm{H}_{3} \mathrm{O}^{+} \)and \( \mathrm{CH}_{3} \mathrm{CO}_{2}^{-} \)at equilibrium, and then answer the following questions.