Home /
Expert Answers /
Physics /
problem-5-a-2-l-bottle-of-coke-rho-coke-1042-k-g-m-3-is-327-mm-tall-fig-5-as-soon-as-yo-pa477
(Solved): Problem 5. A 2-L bottle of coke (\rho coke = 1042 k(g)/(m)3 ) is 327-mm tall (Fig. 5). As soon as yo ...
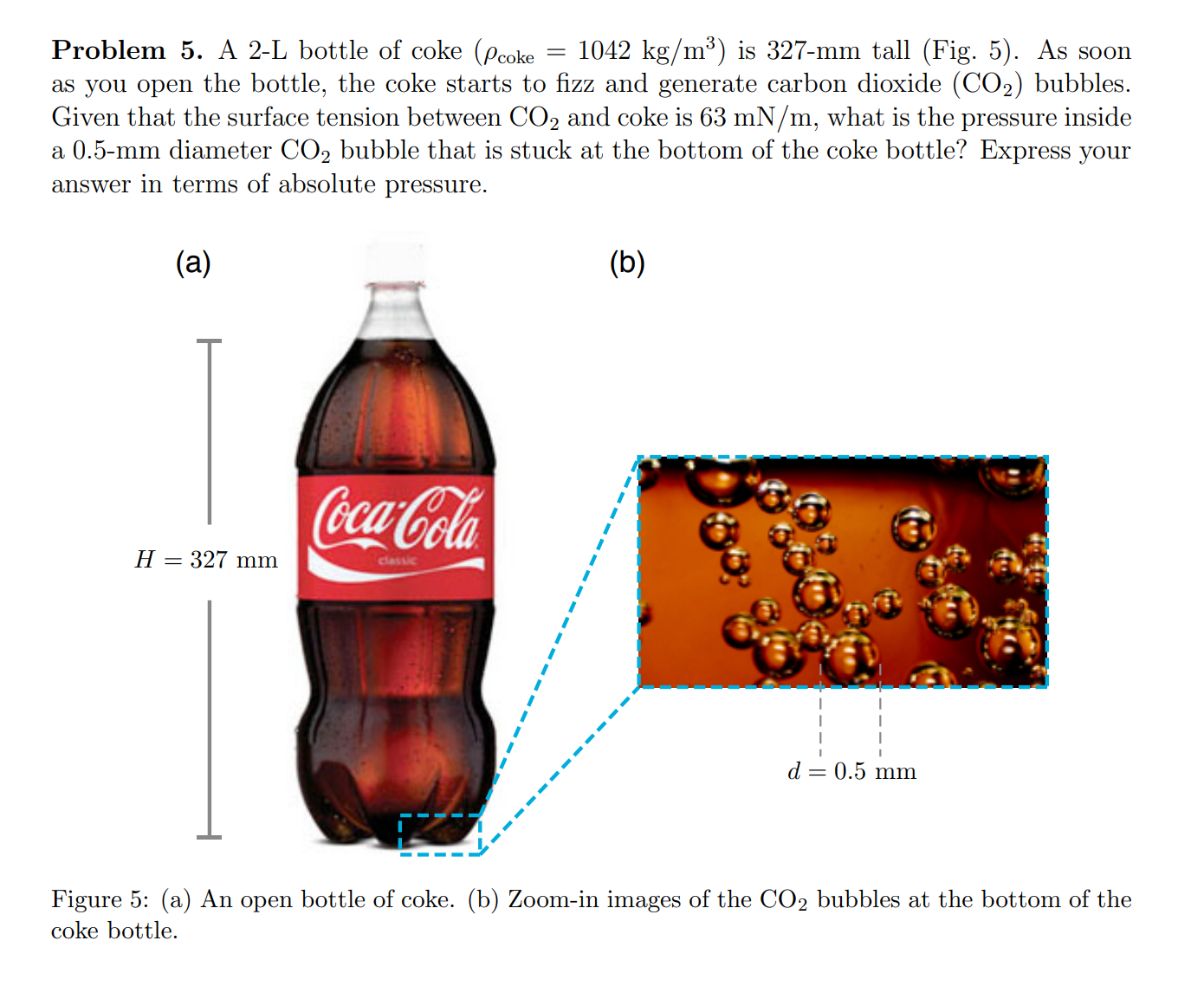
Problem 5. A 2-L bottle of coke (\rho coke = 1042 k(g)/(m)3
) is 327-mm tall (Fig. 5). As soon
as you open the bottle, the coke starts to fizz and generate carbon dioxide (CO2) bubbles.
Given that the surface tension between CO2 and coke is 63 m(N)/(m), what is the pressure inside
a 0.5-mm diameter CO2 bubble that is stuck at the bottom of the coke bottle? Express your
answer in terms of absolute pressure.