Home /
Expert Answers /
Chemistry /
preparing-a-given-volume-of-a-solution-that-has-a-specific-molarity-is-a-very-important-skill-for-a-pa990
(Solved): Preparing a given volume of a solution that has a specific molarity is a very important skill for a ...
Preparing a given volume of a solution that has a specific molarity is a very important skill for a chemist. One step in that process is calculating the mass of solute required.
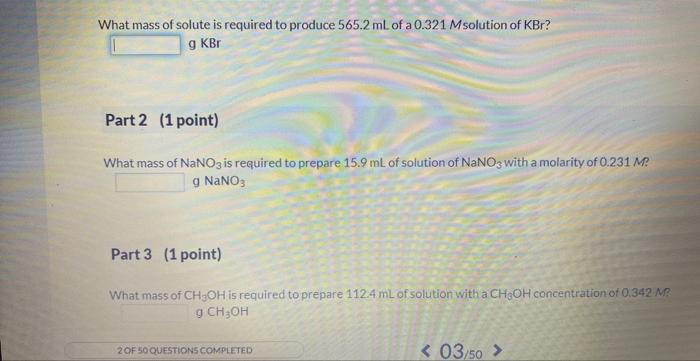

What mass of solute is required to produce \( 565.2 \mathrm{~mL} \) of a \( 0.321 \) Msolution of \( \mathrm{KBr} \) ? \[ \mathrm{g} \mathrm{KBr} \] Part 2 (1 point) What mass of \( \mathrm{NaNO}_{3} \) is required to prepare \( 15.9 \mathrm{~mL} \) of solution of \( \mathrm{NaNO}_{3} \) with a molarity of \( 0.231 \mathrm{M} \) ? \( 9 \mathrm{NaNO}_{3} \) Part 3 (1 point) What mass of \( \mathrm{CH}_{3} \mathrm{OH} \) is required to prepare \( 112.4 \mathrm{~mL} \) of solution with a \( \mathrm{CH}_{3} \mathrm{OH} \) concentration of \( 0.342 . \mathrm{M} \) ? \( \mathrm{g} \mathrm{CH}_{3} \mathrm{OH} \)