Home /
Expert Answers /
Anatomy and Physiology /
predict-whether-each-of-the-following-is-an-ionic-polar-covalent-or-nonpolar-covalent-compound-pa455
(Solved): ??? Predict whether each of the following is an ionic, polar covalent, or nonpolar covalent compound ...
???
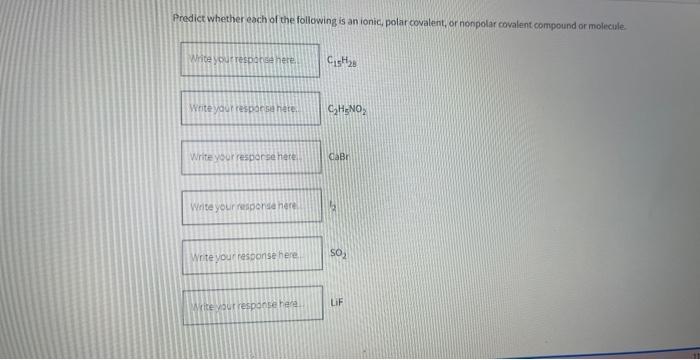

Predict whether each of the following is an ionic, polar covalent, or nonpolar covalent compound or molecale \[ \mathrm{C}_{-15} \mathrm{H}_{28} \] \[ \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NO}_{2} \] CaBr \( \mathrm{SO}_{2} \) LiF
Expert Answer
Ionic bond is formed by complete transfer of electrons between a metal (electropositive) and a non metal(electronegative). Covalent bond is formed by sharing of electrons by both the co species.It is formed between two non met