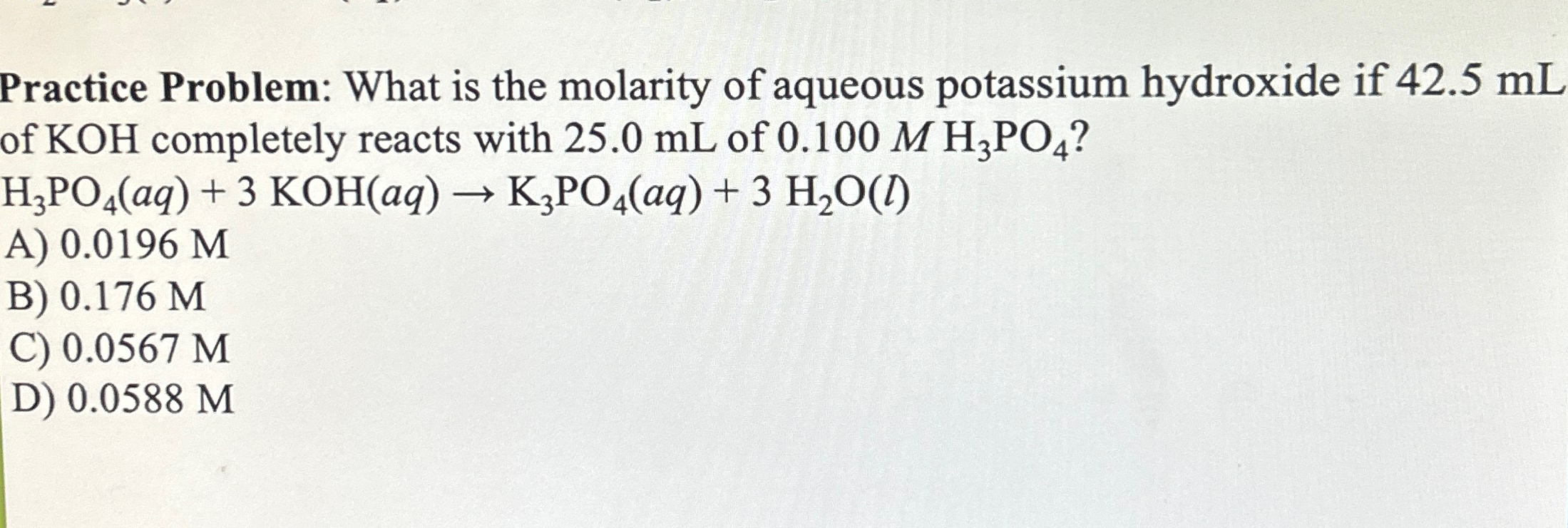Home /
Expert Answers /
Chemistry /
practice-problem-what-is-the-molarity-of-aqueous-potassium-hydroxide-if-42-5ml-of-koh-completely-re-pa124
(Solved): Practice Problem: What is the molarity of aqueous potassium hydroxide if 42.5mL of KOH completely re ...
Practice Problem: What is the molarity of aqueous potassium hydroxide if
42.5mLof
KOHcompletely reacts with
25.0mLof
0.100MH_(3)PO_(4)?
H_(3)PO_(4)(aq)+3KOH(aq)->K_(3)PO_(4)(aq)+3H_(2)O(l)A)
0.0196MB)
0.176MC)
0.0567MD)
0.0588M