Home /
Expert Answers /
Chemistry /
pls-help-with-this-worksheet-data-table-1-data-table-2-b-look-up-the-vapor-pressure-of-water-tabl-pa516
(Solved): pls help with this worksheet DATA TABLE 1 DATA TABLE 2 b) Look up the vapor pressure of water (Tabl ...
pls help with this worksheet
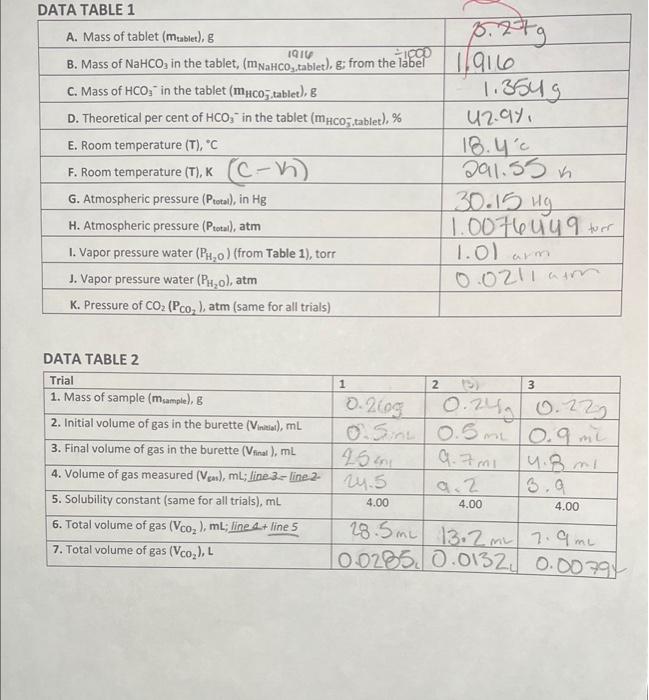



DATA TABLE 1 DATA TABLE 2
b) Look up the vapor pressure of water (Table 1) at your measured temperature. Convert this to atmospheres using dimensional analysis, ( \( 760.0 \) torr \( =1 \) atmosphere). Pwater vaper \( (\mathrm{atm})= \) c) Solve the formula above for \( \mathrm{P}_{\mathrm{CO}_{2}} \) and calculate the pressure of carbon dioxide generated in atmospheres (This will be the same in all three trials since neither temperature nor atmospheric pressure changed during the experiment). \[ \mathrm{P}_{\mathrm{CO}_{2}}=\mathrm{P}_{\text {total }}-\mathrm{P}_{\text {water vapor }} \] \[ P_{\mathrm{CO}_{2}}= \] 4. Calculate the number of moles, generated in each trial, of \( \mathrm{CO}_{2}\left(\mathrm{n}_{\mathrm{CO}_{2}}\right) \) using the ideal gas law \( (\mathrm{R}=0.08206 \mathrm{Latm} / \mathrm{mol} \mathrm{K}): \mathrm{P}_{\mathrm{CO}_{2}} \mathrm{~V}_{\mathrm{CO}_{2}}=\mathrm{n}_{\mathrm{CO}_{2}} \mathrm{RT} \), which rearranges to \[ \mathbf{n}_{\mathrm{CO}_{2}}=\frac{\mathrm{P}_{\mathrm{CO}_{2}} \mathrm{~V}_{\mathrm{CO}_{2}}}{\mathrm{RT}} \] Trial 1: \( \mathrm{n}_{\mathrm{CO}_{2}}= \) Trial 2: \( \mathbf{n}_{\mathrm{CO}_{2}}= \) Trial 3: \( \mathbf{n}_{\mathrm{CO}_{2}}= \) NOTE: \( P_{\mathrm{CO}_{2}}, \mathrm{R} \), and \( \mathrm{T} \) are the same for all trials. \( \mathrm{V}_{\mathrm{CO}_{2}} \) is from Data Table 2, Line 7. 5. Calculate the mass of \( \mathrm{HCO}_{3}^{-2} \) in your sample using the formula \[ m_{\mathrm{HCO}_{3}^{-}}=n_{\mathrm{HCO}_{3}^{-}} \times \mathrm{MM}_{\mathrm{HCO}_{3}^{-}} \]
Where \( n_{\mathrm{HCO}_{3}} \) is the number of moles of moles of \( \mathrm{HCO}_{3}^{-} ;\left(\mathrm{n}_{\mathrm{HCO}_{3}^{-}}=\mathrm{n}_{\mathrm{CO}_{2}}\right): \mathrm{MM}_{\mathrm{HCO}_{3}^{-}} \)is the molar mass of the \( \mathrm{HCO}_{3}^{-}(\mathrm{g} / \mathrm{mol}) \). Trial 1: \( \mathrm{m}_{\mathrm{Hco}}^{-} \mathrm{=}= \) Trial 2: \( \mathrm{m}_{\mathrm{HCO}} \mathrm{=}= \) Trial 3: \( \mathrm{m}_{\mathrm{HCO}_{3}}= \) 6. Calculate the mass percent bjcarbonate (\%ncos) in your samples for each trial using formula: Trial 1: \( \%_{H \mathrm{CO}_{3}^{-}}= \) Trial 2: \( \%_{\mathrm{HCO}_{3}^{-}}= \) Trial 3: \( \%_{H \mathrm{CO}_{3}^{-}}= \) Average the value of \( \%_{\mathrm{HCO}_{3}^{-}} \)for all three trials. Actual (average): \( \%_{\mathrm{HCO}_{3}^{-}}= \) 7. Calculate \% error using the equation: \%error \( \mathrm{HCO}_{3}^{-}= \) \( \left|\frac{9 \text { theoretical-\%actual (average) }}{\text { \%theorectical }}\right| \) \%error \( _{\mathrm{HCO}_{3}^{-}}= \)
Expert Answer
Temperature=18.4°C =(273+18.4)K = 291.4 K B. Vapor pressure of water at 18.4°C = 15.897 mmHg = 15.897 mmHg÷760mmHg/atm = 0.021 atm C. PCO2 = PTotal - PCO2 =( 1.0076 - 0.021) atm = 0.9866 atm 4. Trial -1