Home /
Expert Answers /
Chemistry /
please-help-when-the-following-redox-reaction-is-balanced-in-basic-solution-what-is-the-coefficient-pa285
(Solved): please help When the following redox reaction is balanced in basic solution, what is the coefficient ...
please help
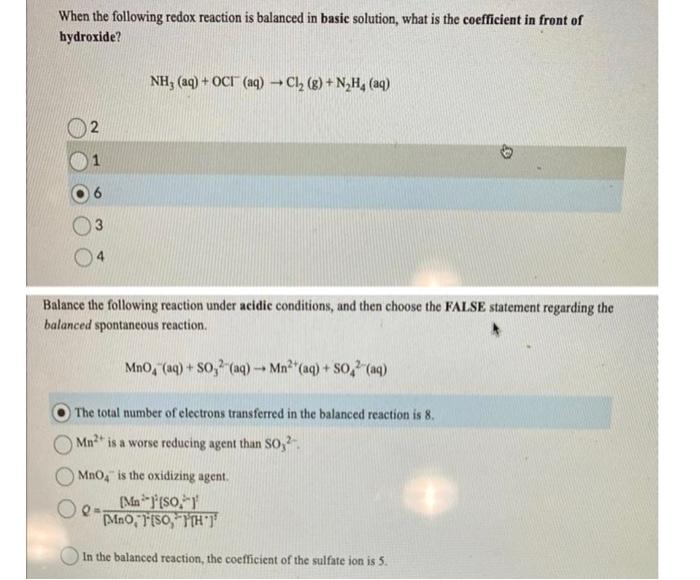

When the following redox reaction is balanced in basic solution, what is the coefficient in front of hydroxide? \[ \mathrm{NH}_{3}(\mathrm{aq})+\mathrm{OCl}(\mathrm{aq}) \rightarrow \mathrm{Cl}_{2}(\mathrm{~g})+\mathrm{N}_{2} \mathrm{H}_{4}(\mathrm{aq}) \] 2 1 6 3 4 Balance the following reaction under acidic conditions, and then choose the FALSE statement regarding the balanced spontaneous reaction. \[ \mathrm{MnO}_{4}^{-}(\mathrm{aq})+\mathrm{SO}_{3}^{2-}(\mathrm{aq}) \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+\mathrm{SO}_{4}^{2-}(\mathrm{aq}) \] The total number of electrons transferred in the balanced reaction is 8 . \( \mathrm{Mn}^{2+} \) is a worse reducing agent than \( \mathrm{SO}_{3}^{2} \). \( \mathrm{MnO}_{4}{ }^{-} \)is the oxidizing agent. \[ Q=\frac{\left[\mathrm{Mn}^{2-j}\right]^{j}\left[\mathrm{SO}_{4}^{-}\right]^{2}}{\left[\mathrm{MnO}_{4}^{-}\right]^{2}\left[\mathrm{SO}_{4}^{2} \mathrm{H}^{\prime} \mathrm{H}^{+}\right]^{3}} \] In the balanced reaction, the coefficient of the sulfate ion is 5 .