Home /
Expert Answers /
Chemistry /
please-help-solve-the-question-hint-the-answers-are-not-ph-1-09-or-0-079-m-for-equilibrium-of-h-pa693
(Solved): Please help solve the question. Hint : The answers are not pH = 1.09 or 0.079 M for equilibrium of H ...
Please help solve the question. Hint : The answers are not pH = 1.09 or 0.079 M for equilibrium of H2SO4
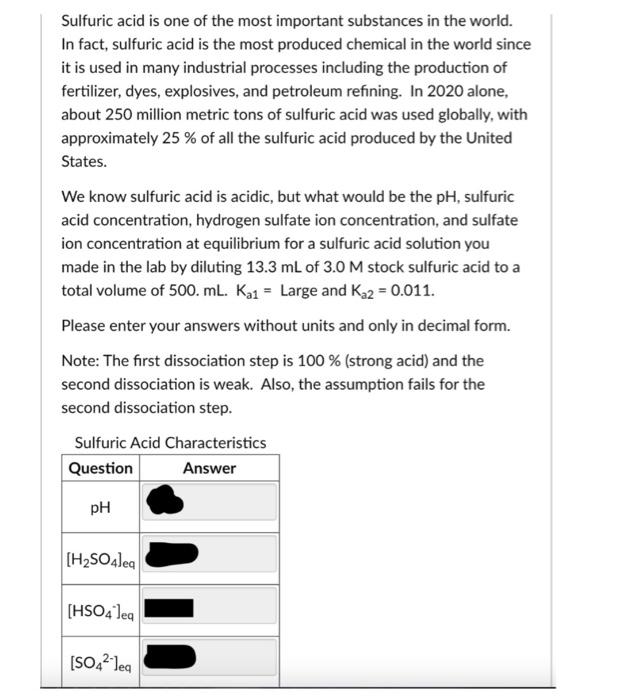

Sulfuric acid is one of the most important substances in the world. In fact, sulfuric acid is the most produced chemical in the world since it is used in many industrial processes including the production of fertilizer, dyes, explosives, and petroleum refining. In 2020 alone, about 250 million metric tons of sulfuric acid was used globally, with approximately \( 25 \% \) of all the sulfuric acid produced by the United States. We know sulfuric acid is acidic, but what would be the \( \mathrm{pH} \), sulfuric acid concentration, hydrogen sulfate ion concentration, and sulfate ion concentration at equilibrium for a sulfuric acid solution you made in the lab by diluting \( 13.3 \mathrm{~mL} \) of \( 3.0 \mathrm{M} \) stock sulfuric acid to a total volume of \( 500 . \mathrm{mL} . \mathrm{K}_{\mathrm{a} 1}= \) Large and \( \mathrm{K}_{\mathrm{a} 2}=0.011 \). Please enter your answers without units and only in decimal form. Note: The first dissociation step is \( 100 \% \) (strong acid) and the second dissociation is weak. Also, the assumption fails for the second dissociation step. Sulfuric Acid Characteristics