Home /
Expert Answers /
Chemistry /
please-help-nbsp-what-is-the-kb-for-the-conjugate-base-of-an-unknown-acid-with-a-mathrm-k-3-pa895
(Solved): please help What is the Kb for the conjugate base of an unknown acid with a \( \mathrm{K} 3 \) ...
please help
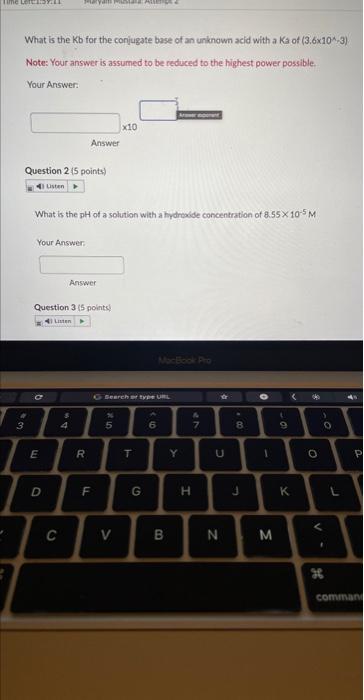



What is the Kb for the conjugate base of an unknown acid with a \( \mathrm{K} 3 \) of \( \left(3.6 \times 10^{*}, 3\right) \). Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: Answer Question 2 (5 points) What is the \( \mathrm{pH} \) of a solution with a hydroxide concentration of \( 8.55 \times 10^{-5} \mathrm{M} \) Your Answer: Answer Question 3 (5 points)
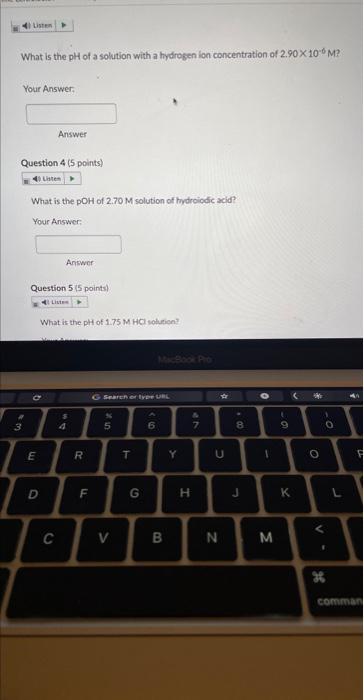
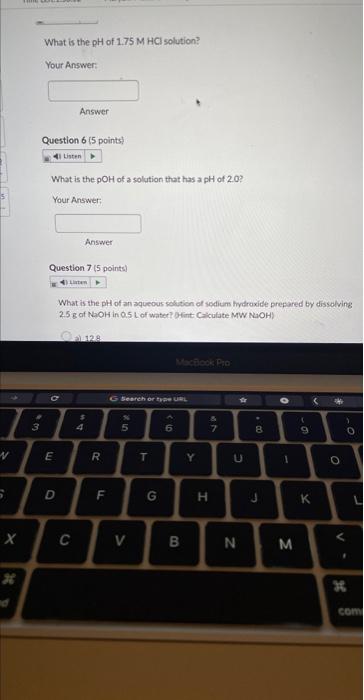
What is the \( \mathrm{pH} \) of a solution with a hydrogen ion concentration of \( 2.90 \times 10^{-6} \mathrm{M} \) ? Your Answer: Answer Question 4 ( 5 points) What is the pOH of \( 2.70 \mathrm{M} \) solution of tydroiodic acid? Your Answer: Answer Question 5 ( 5 points) What is the pH of \( 1.75 \mathrm{M} \mathrm{HCl} \) sotien?
What is the \( \mathrm{pOH} \) of a solution that has a pH of \( 2.0 \) ? Your Answer; Answer Question 7 ( 5 points) What is the pH of an aqueous selition of sedicm frytraxide prepared by dissolving \( 2.5 \mathrm{~g} \) of \( \mathrm{WaOH} \) in \( 0.5 \mathrm{~L} \) of water? Dint (Ciculate MWW NiOH)