Home /
Expert Answers /
Chemistry /
please-help-nbsp-1-draw-a-distillation-curve-for-fractional-distillation-experiment-2-why-is-fra-pa404
(Solved): please help. 1) Draw a distillation curve for fractional distillation experiment 2) Why is fra ...
please help.



1) Draw a distillation curve for fractional distillation experiment 2) Why is fractional distillation better than a simple distillation? 3) What is the purpose of the copper sponge? 4) What is the identity of your unknown mixture? 5) (a) What is the effect on the boiling point of a solution (e.g., water) produced by a soluble nonvolatile substance (e.g., sodium chloride)? What is the effect of an insoluble substance such as sand or charcoal? What is the temperature of the vapor above these two boiling solutions? (b) Why is better separation of two liquids achieved by slow ratner than fast distillation? (c) What effect does the reduction of atmospheric pressure have on the boiling point? Can cyclohexane and toluene be separated if the external pressure is \( 350 \mathrm{~mm} \mathrm{Hg} \) instead of 760 \( \mathrm{mmHg} \) ? 6) In addition, answer following post-lab questions. (a) The observed melting point ranges for pure cinnamic acid and urea each were \( 132-133^{\circ} \mathrm{C} \). However, the melting point of a mixture of urea and cinnamic acid was observed to be 122 \( 127^{\circ} \mathrm{C} \). Explain for this discrepancy in the melting points.
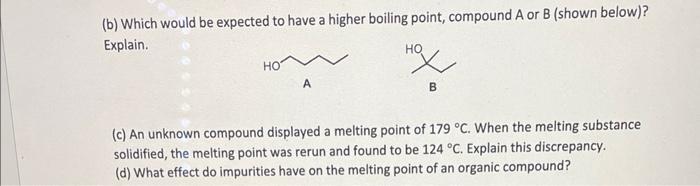
(b) Which would be expected to have a higher boiling point, compound A or B (shown below)? Explain. (c) An unknown compound displayed a melting point of \( 179^{\circ} \mathrm{C} \). When the melting substance solidified, the melting point was rerun and found to be \( 124^{\circ} \mathrm{C} \). Explain this discrepancy. (d) What effect do impurities have on the melting point of an organic compound?