Home /
Expert Answers /
Chemistry /
please-answer-question-calculate-the-enthalpy-change-for-the-production-of-2-moles-of-calcium-chlor-pa601
(Solved): please answer question! Calculate the enthalpy change for the production of 2 moles of calcium chlor ...
please answer question!
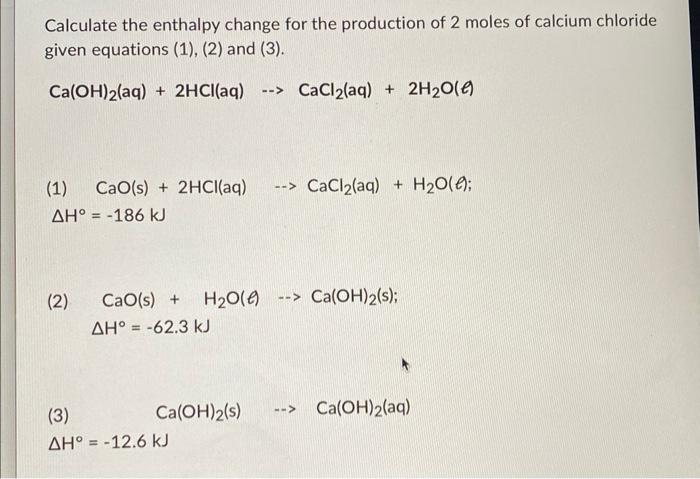

Calculate the enthalpy change for the production of 2 moles of calcium chloride given equations (1), (2) and (3). \[ \mathrm{Ca}(\mathrm{OH})_{2}(\mathrm{aq})+2 \mathrm{HCl}_{(a q)} \rightarrow \mathrm{CaCl}_{2}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\ell) \] (1) \( \mathrm{CaO}(\mathrm{s})+2 \mathrm{HCl}(\mathrm{aq}) \quad \cdots \mathrm{CaCl}_{2}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}\left(\ell^{\prime}\right. \); \[ \Delta H^{\circ}=-186 \mathrm{~kJ} \] ( 2 ) \[ \begin{array}{c} \mathrm{CaO}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\ell) \cdots \mathrm{Ca}(\mathrm{OH})_{2}(\mathrm{~s}) ; \\ \Delta \mathrm{H}^{\circ}=-62.3 \mathrm{~kJ} \end{array} \] \[ \begin{array}{llll} \text { (3) } \quad \mathrm{Ca}(\mathrm{OH})_{2}(\mathrm{~s}) \quad \cdots \mathrm{Ca}(\mathrm{OH})_{2}(\mathrm{aq}) \\ \Delta \mathrm{H}^{\circ}=-12.6 \mathrm{~kJ} \end{array} \]