Home /
Expert Answers /
Chemistry /
please-answer-all-thank-you-nbsp-draw-the-structure-s-of-the-major-organic-product-s-of-the-follo-pa649
(Solved): please answer all thank you Draw the structure(s) of the major organic product(s) of the follo ...
please answer all thank you
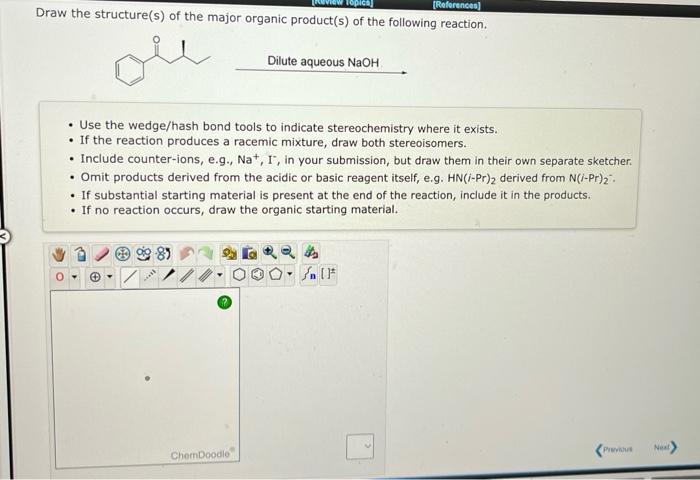





Draw the structure(s) of the major organic product(s) of the following reaction. Dilute aqueous \( \mathrm{NaOH} \) - Use the wedge/hash bond tools to indicate stereochemistry where it exists. - If the reaction produces a racemic mixture, draw both stereoisomers. - Include counter-ions, e.g., \( \mathrm{Na}^{+}, \mathrm{I}^{-} \), in your submission, but draw them in their own separate sketcher. - Omit products derived from the acidic or basic reagent itself, e.g. \( \mathrm{HN}(i-\mathrm{Pr})_{2} \) derived from \( \mathrm{N}(i-\mathrm{Pr})_{2} \) : - If substantial starting material is present at the end of the reaction, include it in the products. - If no reaction occurs, draw the organic starting material.
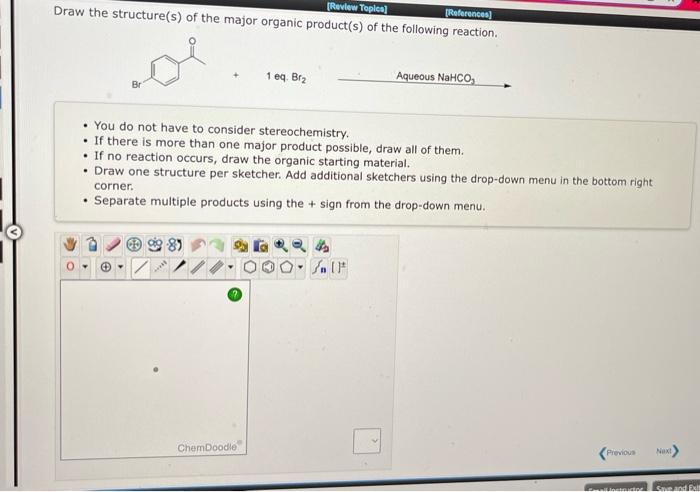
Draw the structure(s) of the major organic product(s) of the following reaction. 1 eq. \( \mathrm{Br}_{2} \) Aqueous \( ^{2} \mathrm{NaHCO}_{3} \longrightarrow \) - You do not have to consider stereochemistry. - If there is more than one major product possible, draw all of them. - If no reaction occurs, draw the organic starting material. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple products using the + sign from the drop-down menu.
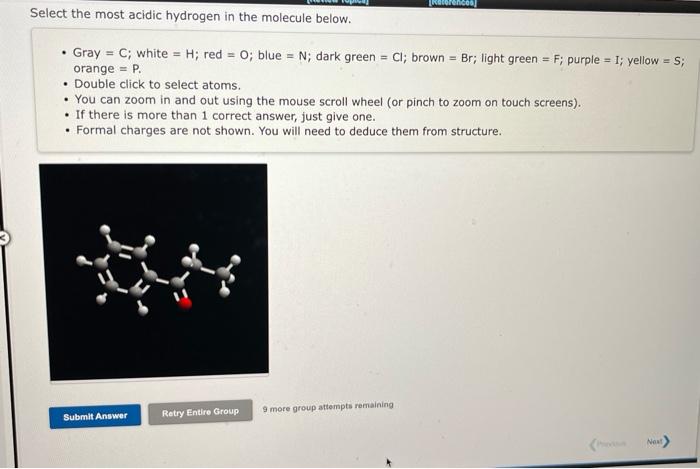
- Gray \( =\mathrm{C} ; \) white \( =\mathrm{H} \); red \( =\mathrm{O} \); blue \( =\mathrm{N} \); dark green \( =\mathrm{Cl} ; \) brown \( =\mathrm{Br} \); light green \( =\mathrm{F} \); purple = I; yellow = ; ; orange \( = \) P. - Double click to select atoms. - You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). - If there is more than 1 correct answer, just give one. - Formal charges are not shown. You will need to deduce them from structure.
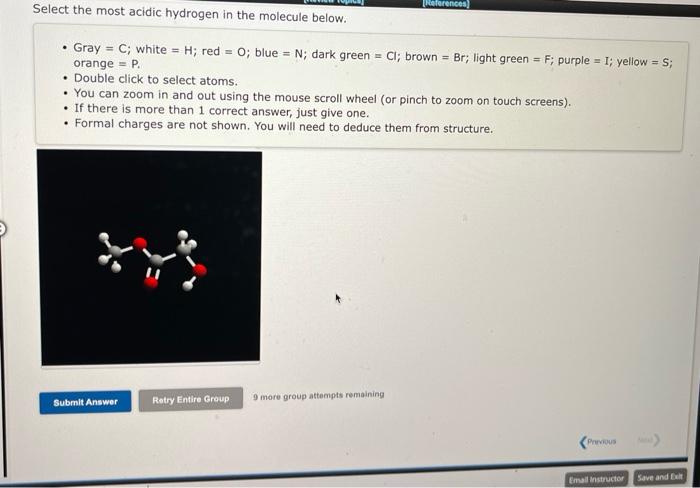
- Gray \( =\mathrm{C} ; \) white \( =\mathrm{H} \); red \( =\mathrm{O} \); blue \( =\mathrm{N} \); dark green \( =\mathrm{Cl} ; \) brown \( =\mathrm{Br} \); light green \( =\mathrm{F} \); purple \( =\mathrm{I} \); yellow \( =\mathrm{S} \); orange \( =P \). - Double click to select atoms. - You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). - If there is more than 1 correct answer, just give one. - Formal charges are not shown. You will need to deduce them from structure.
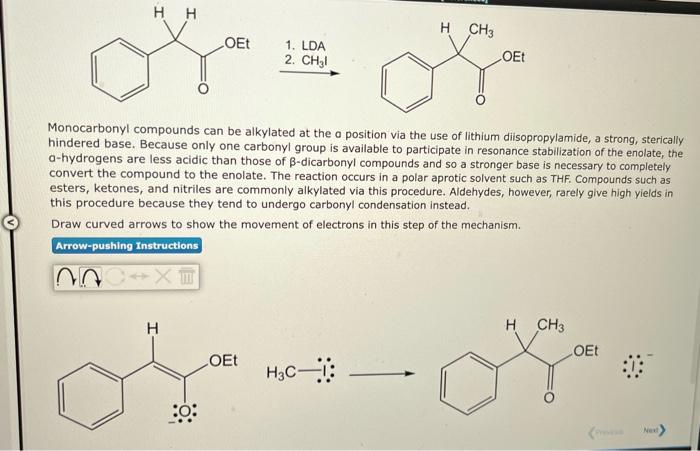
1. LDA \( \stackrel{\text { 2. } \mathrm{CH}_{3} \mathrm{I}}{\longrightarrow} \) Monocarbonyl compounds can be alkylated at the a position via the use of lithium diisopropylamide, a strong, sterically hindered base. Because only one carbonyl group is available to participate in resonance stabilization of the enolate, the \( \mathrm{a} \)-hydrogens are less acidic than those of \( \beta \)-dicarbonyl compounds and so a stronger base is necessary to completely convert the compound to the enolate. The reaction occurs in a polar aprotic solvent such as THF. Compounds such as esters, ketones, and nitriles are commonly alkylated via this procedure. Aldehydes, however, rarely give high yields in this procedure because they tend to undergo carbonyl condensation instead. Draw curved arrows to show the movement of electrons in this step of the mechanism.