Home /
Expert Answers /
Chemistry /
please-answer-all-4-parts-of-this-question-nbsp-1-propyne-reacts-with-diisiamylborane-followed-pa867
(Solved): Please answer all 4 parts of this question. 1-Propyne reacts with diisiamylborane, followed ...
Please answer all 4 parts of this question.
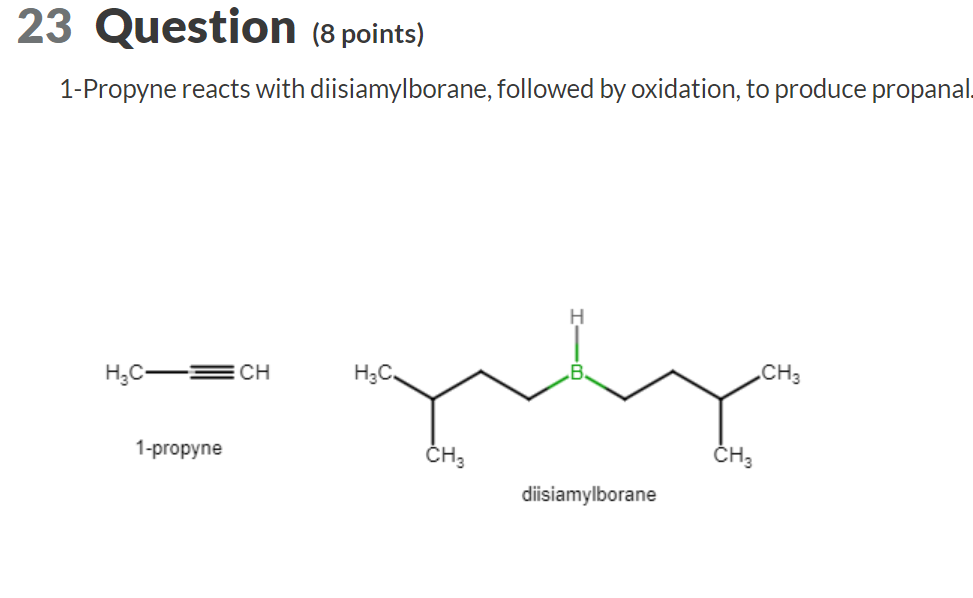
1-Propyne reacts with diisiamylborane, followed by oxidation, to produce propana \( \mathrm{H}_{3} \mathrm{C}=\mathrm{CH} \) 1-propyne diisiamylborane
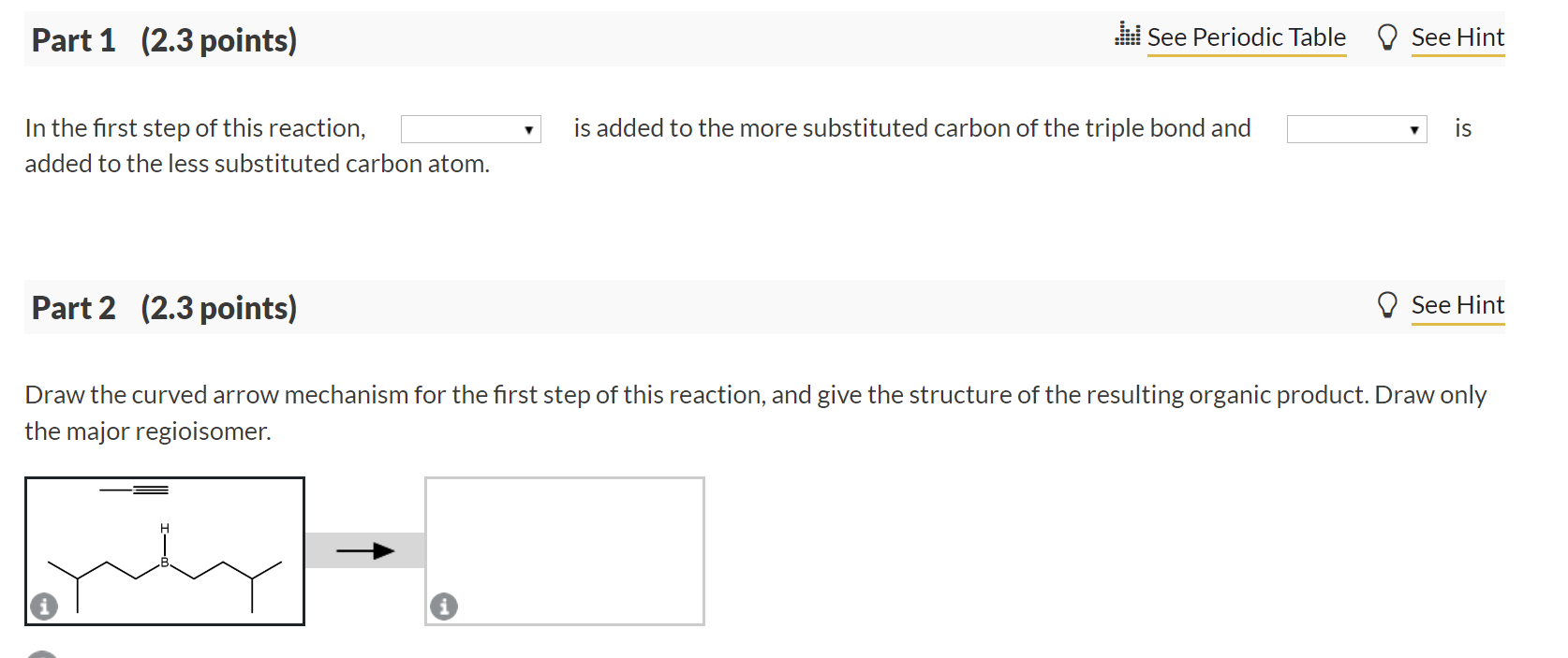
In the first step of this reaction, is added to the more substituted carbon of the triple bond and is added to the less substituted carbon atom. Part 2 (2.3 points) Draw the curved arrow mechanism for the first step of this reaction, and give the structure of the resulting organic product. Draw only the major regioisomer.
The organoborane formed in step 1 is oxidized by \( \mathrm{H}_{2} \mathrm{O}_{2} \) in the presence of \( \mathrm{OH}^{-} \)in the next step of the reaction. Which of the following options correctly describe this process? Choose one or more: A. \( \mathrm{OH}^{-} \)must attack from the face of the alkene opposite the \( -\mathrm{BR} 2 \) group. B. OH replaces \( -\mathrm{BR} 2 \) in this step. C. OH replaces \( \mathrm{H} \) in this step. D. Configuration at the carbon-bearing \( B \) is retained in the reaction. E. -BR2 acts as a leaving group in this step.
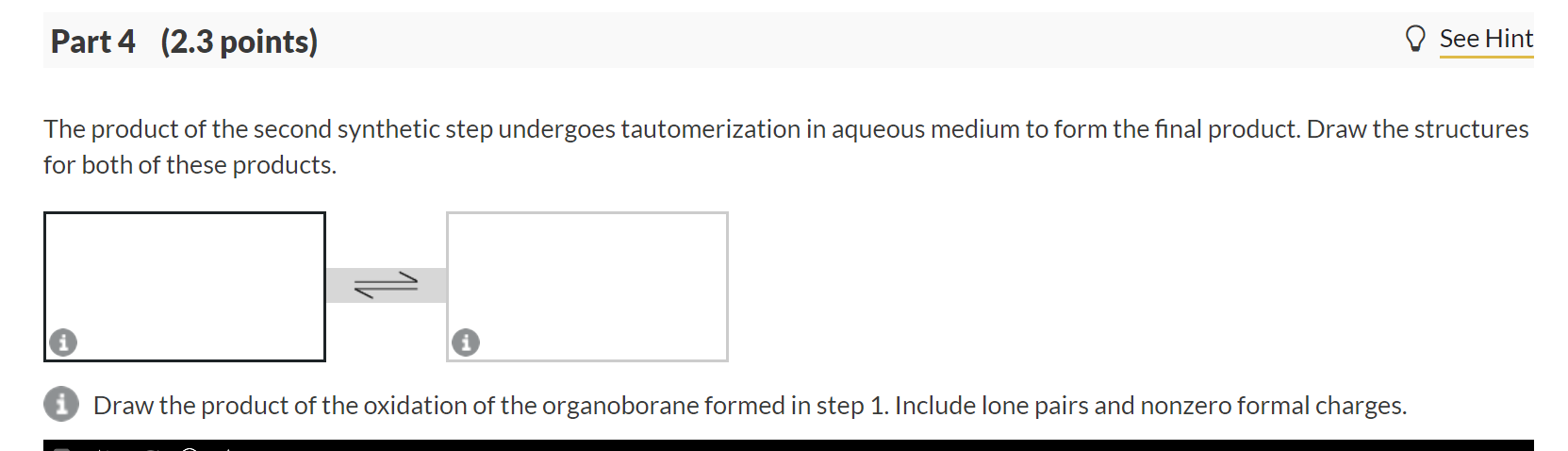
The product of the second synthetic step undergoes tautomerization in aqueous medium to form the final product. Draw the structures for both of these products.