Home /
Expert Answers /
Chemistry /
pi-bonding-a-bond-arises-from-34-sideways-34-overiap-of-two-parallel-p-orbitals-the-elect-pa314
(Solved): \( \pi \) Bonding \( A \) bond arises from "sideways" overiap of two parallel p orbitals. The elect ...
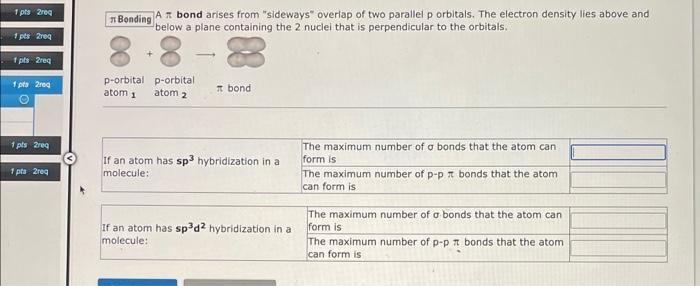
\( \pi \) Bonding \( A \) bond arises from "sideways" overiap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals,
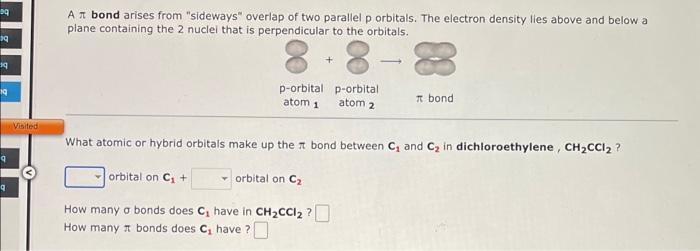
A \( \pi \) bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclel that is perpendicular to the orbitals. What atomic or hybrid orbitals make up the \( \pi \) bond between \( \mathrm{C}_{1} \) and \( \mathrm{C}_{2} \) in dichloroethylene, \( \mathbf{C H}_{2} \mathrm{CCl}_{2} \) ? orbital on \( \mathbf{C}_{1}+\quad \) orbital on \( \mathbf{C}_{2} \) How many \( \sigma \) bonds does \( \mathrm{C}_{1} \) have in \( \mathrm{CH}_{2} \mathrm{CCl}_{2} \) ? How many \( \pi \) bonds does \( \boldsymbol{C}_{1} \) have ?
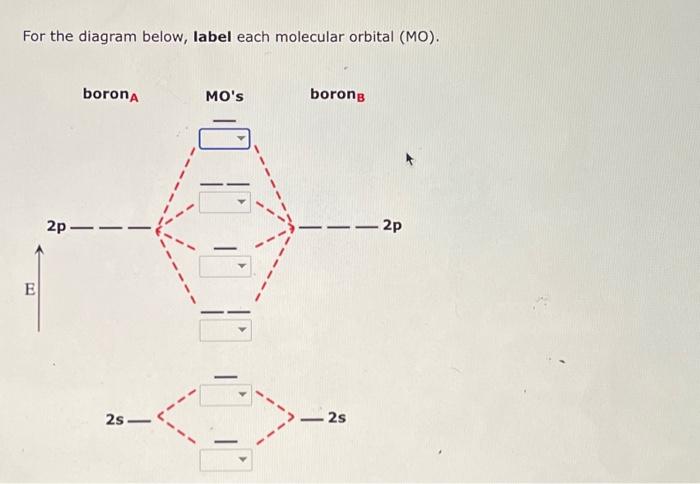
For the diagram below, label each molecular orbital (MO).