Home /
Expert Answers /
Physics /
part-a-rutherford-39-s-scattering-experiments-gave-the-first-indications-that-an-atom-consists-of-a--pa965
(Solved): Part A Rutherford's scattering experiments gave the first indications that an atom consists of a s ...
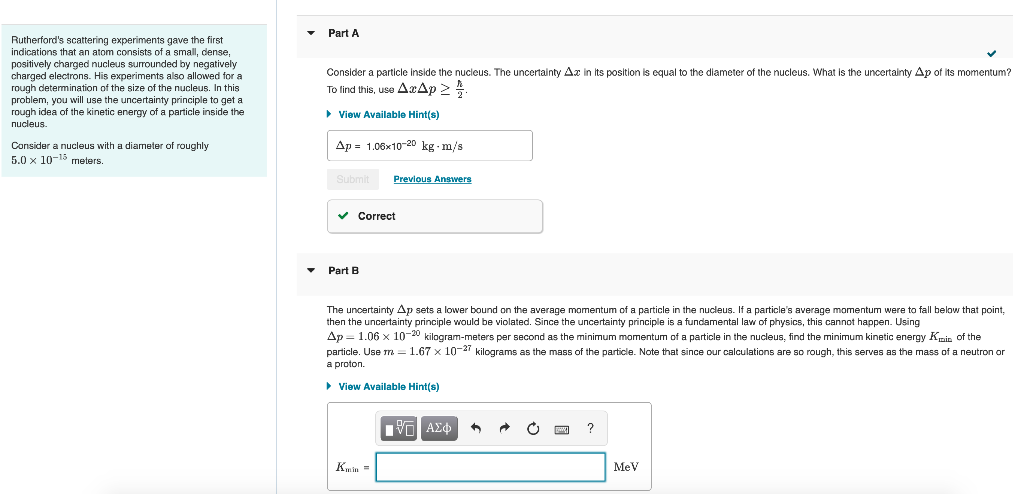
Part A Rutherford's scattering experiments gave the first indications that an atom consists of a small, dense, positively charged nucleus surrounded by negatively charged electrons. His experiments also allowed for a rough determination of the size of the nucleus. In this problem, you will use the uncertainty principle to get a rough idea of the kinetic energy of a particle inside the nucleus. Consider a nucleus with a diameter of roughly 5.0 x 10-15 meters. Consider a particle inside the nucleus. The uncertainty Az in its position is equal to the diameter of the nucleus. What is the uncertainty Ap of its momentum? To find this, use AcAp> View Available Hint(s) Ap = 1.06x10-20 kg.m/s Submit Previous Answers ? Correct Part B The uncertainty Ap sets a lower bound on the average momentum of a particle in the nucleus. If a particle's average momentum were to fall below that point, then the uncertainty principle would be violated. Since the uncertainty principle is a fundamental law of physics, this cannot happen. Using Ap=1.06 x 10-20 kilogram-meters per second as the minimum momentum of a particle in the nucleus, find the minimum kinetic energy Knit of the particle. Use m = 1.67 x 10-27 kilograms as the mass of the particle. Note that since our calculations are so rough, this serves as the mass of a neutron or a proton View Available Hint(s) EVO AED Kin= MeV