Home /
Expert Answers /
Chemistry /
part-1-lewis-structures-and-molecular-shapes-draw-the-lewis-structure-for-sf6-how-many-lone-pai-pa979
Expert Answer
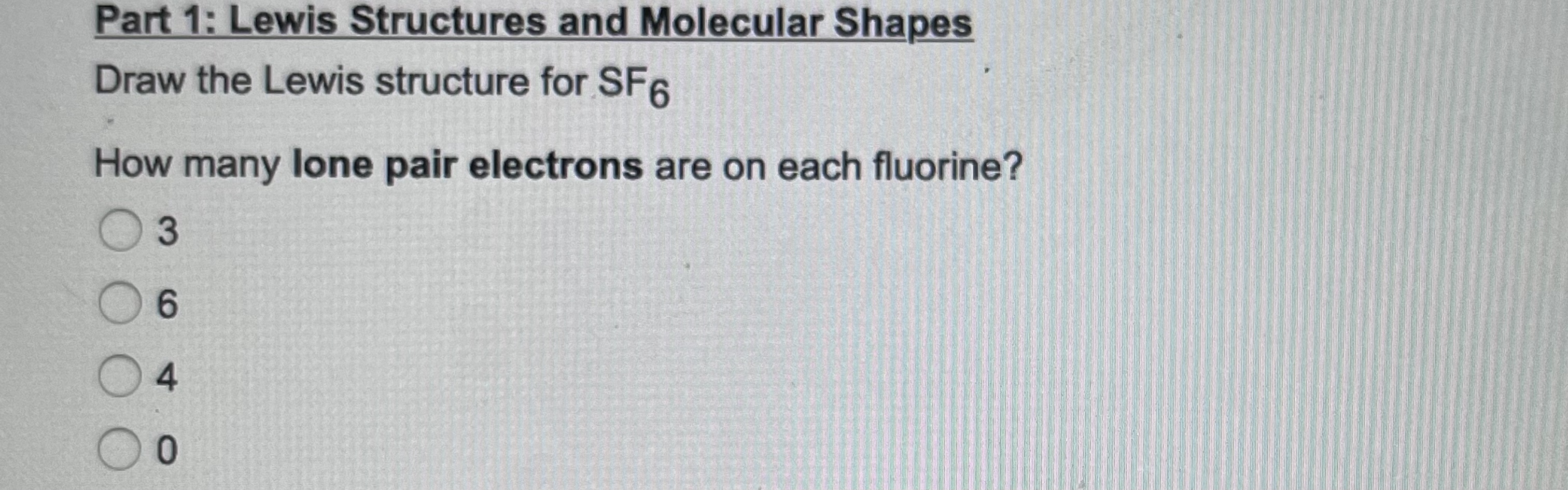
lewis structure of SF6The Lewis structure of SF6 (sulfur hexafluoride) is as follows:Determine the total number of valence electrons: Sulfur has 6 valence electrons, and each fluorine atom contributes 7 valence electrons. Therefore, the total number of valence electrons in SF6 is: