Home /
Expert Answers /
Chemistry /
organic-chemistry-review-need-correct-answers-4-is-the-following-molecule-cis-or-trans-a-cis-b-pa503
(Solved): Organic chemistry review. Need correct answers 4. Is the following molecule cis or trans? a) cis b) ...
Organic chemistry review. Need correct answers
![20. Consider the reaction below. Which side does the equilibrium favor?
\[
\Rightarrow
\]
a) the right because the tert-butyl](https://media.cheggcdn.com/study/6be/6bed33d7-d7f9-4bee-be1d-55751b21ea80/image)



![20. Consider the reaction below. Which side does the equilibrium favor?
\[
\Rightarrow
\]
a) the right because the tert-butyl](https://media.cheggcdn.com/study/6be/6bed33d7-d7f9-4bee-be1d-55751b21ea80/image)

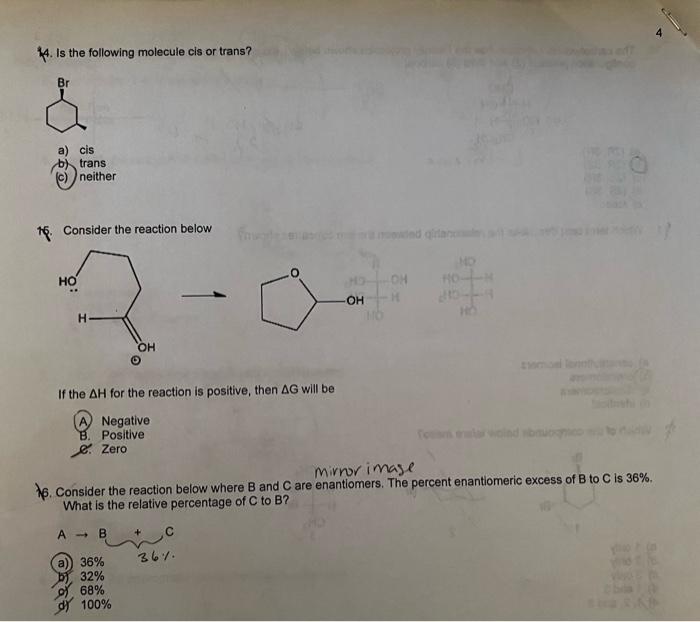
4. Is the following molecule cis or trans? a) cis b) trans (c) neither 16. Consider the reaction below If the \( \Delta H \) for the reaction is positive, then \( \Delta G \) will be A) Negative B. Positive C. Zero 16. Consider the reaction below where \( B \) and \( C \) are enantiomers. The percent enantiomeric excess of B to C is \( 36 \% \). What is the relative percentage of \( C \) to \( B \) ? a) \( 36 \% \) \( 36 \% \) b), \( 32 \% \) g) \( 68 \% \) d) \( 100 \% \)
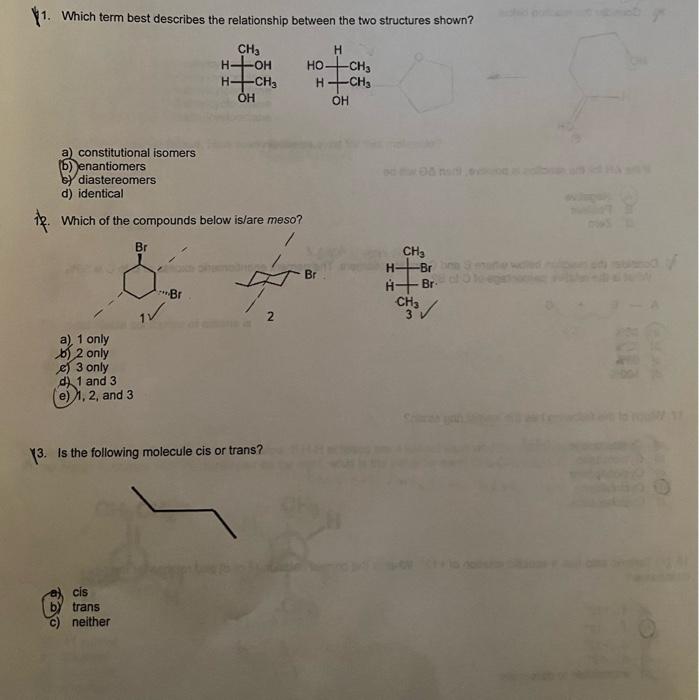
11. Which term best describes the relationship between the two structures shown? a) constitutional isomers (b) enantiomers b) diastereomers d) identical 12. Which of the compounds below is/are meso? a) 1 only b) 2 only c) 3 only d) 1 and 3 e) 1,2 , and 3 (3. Is the following molecule cis or trans? a) cis b) trans c) neither
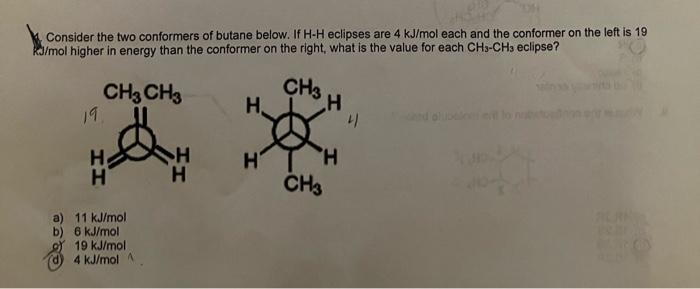
4. Consider the two conformers of butane below. If \( \mathrm{H} \cdot \mathrm{H} \) eclipses are \( 4 \mathrm{~kJ} / \mathrm{mol} \) each and the conformer on the left is 19 \( \mathrm{kJ} / \mathrm{mol} \) higher in energy than the conformer on the right, what is the value for each \( \mathrm{CH}_{3}-\mathrm{CH}_{3} \) eclipse? a) \( 11 \mathrm{~kJ} / \mathrm{mol} \) b) \( 6 \mathrm{~kJ} / \mathrm{mol} \) of \( 19 \mathrm{~kJ} / \mathrm{mol} \) (d) \( 4 \mathrm{~kJ} / \mathrm{mol} \mathrm{A} \)
20. Consider the reaction below. Which side does the equilibrium favor? \[ \Rightarrow \] a) the right because the tert-butyl group is in the axial position b) the right because the tert-butyl group is in the equatorial position c) the left because the tert-butyl group is in the axial position (d) the left because the tert-butyl group is in the equatorial position
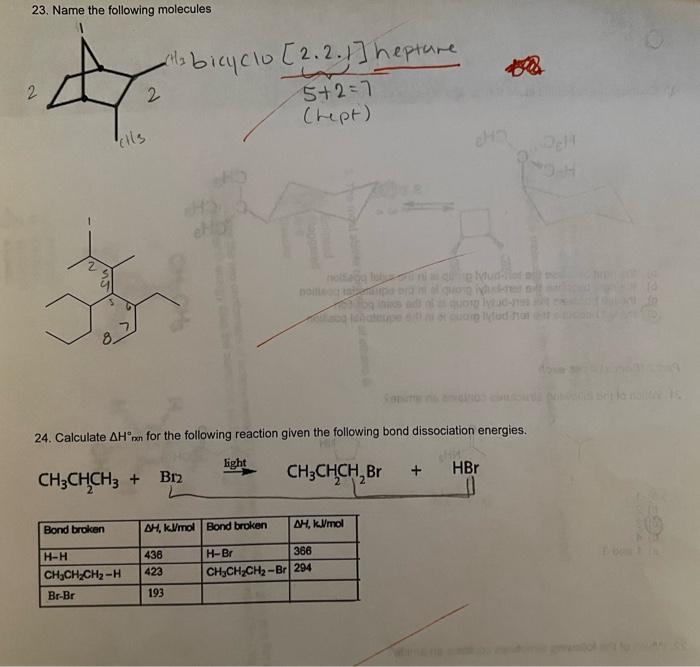
23. Name the following molecules 24. Calculate \( \Delta H^{\circ} \) xn for the following reaction given the following bond dissociation energies. \[ \mathrm{CH}_{3} \mathrm{CHCH}_{2}+\underset{\mathrm{Br}}{2} \stackrel{\text { light }}{\longrightarrow} \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{HBr} \]