Home /
Expert Answers /
Chemistry /
nernst-equation-applied-to-half-reactions-the-nernst-equation-can-be-applied-to-half-reactions-ca-pa223
(Solved): Nernst Equation Applied to Half-Reactions The Nernst equation can be applied to half-reactions. Ca ...
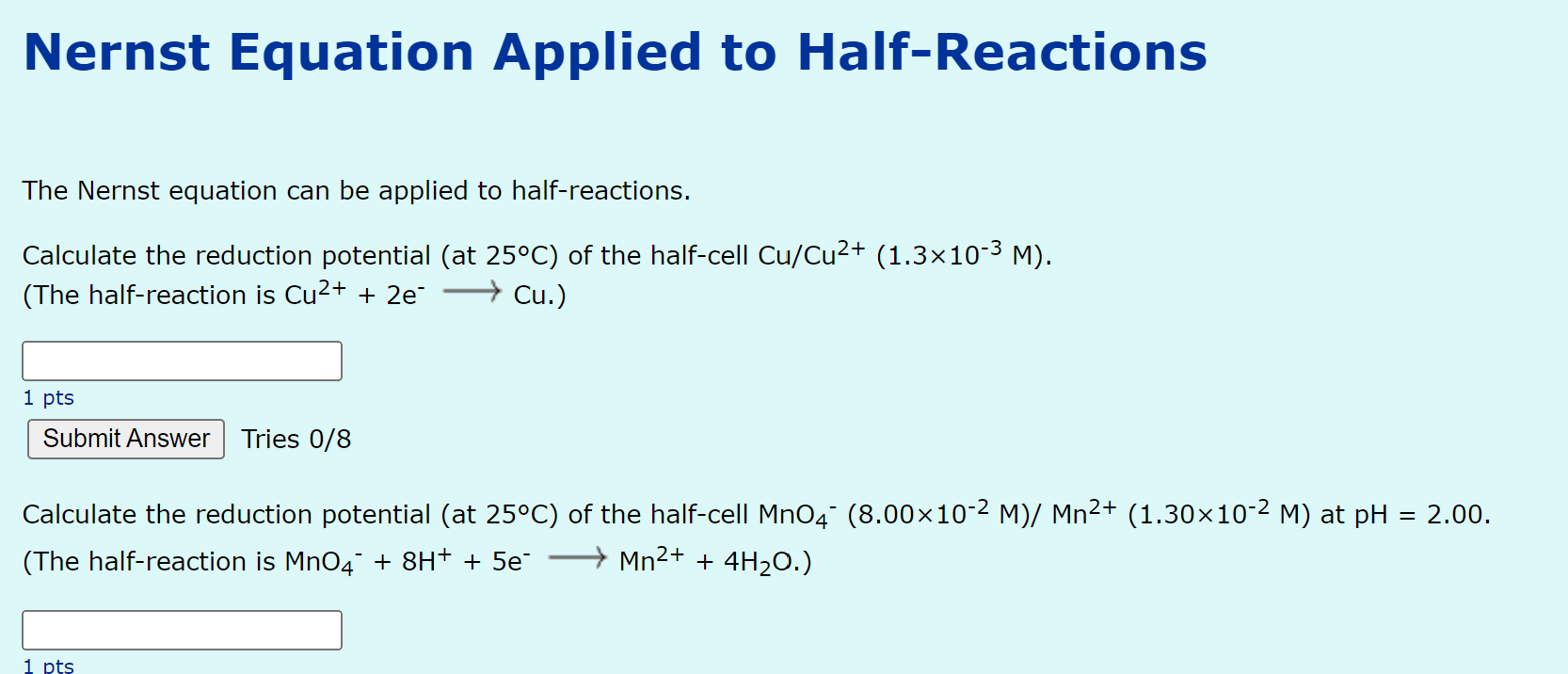
Nernst Equation Applied to Half-Reactions The Nernst equation can be applied to half-reactions. Calculate the reduction potential \( \left(\right. \) at \( \left.25^{\circ} \mathrm{C}\right) \) of the half-cell \( \mathrm{Cu} / \mathrm{Cu}^{2+}\left(1.3 \times 10^{-3} \mathrm{M}\right) \). (The half-reaction is \( \mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \longrightarrow \mathrm{Cu} \).) 1 pts Tries 0/8 Calculate the reduction potential \( \left(\right. \) at \( \left.25^{\circ} \mathrm{C}\right) \) of the half-cell \( \mathrm{MnO}_{4^{-}}\left(8.00 \times 10^{-2} \mathrm{M}\right) / \mathrm{Mn}^{2+}\left(1.30 \times 10^{-2} \mathrm{M}\right) \) at \( \mathrm{pH}=2.00 \). (The half-reaction is \( \mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \longrightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \).)