Home /
Expert Answers /
Chemistry /
nbsp-the-calculation-of-the-area-under-a-1-mathrm-h-nmr-peak-is-pa471
(Solved): The calculation of the area under a \( { }^{1} \mathrm{H} \) NMR peak is ...
The calculation of the area under a \( { }^{1} \mathrm{H} \) NMR peak is called the
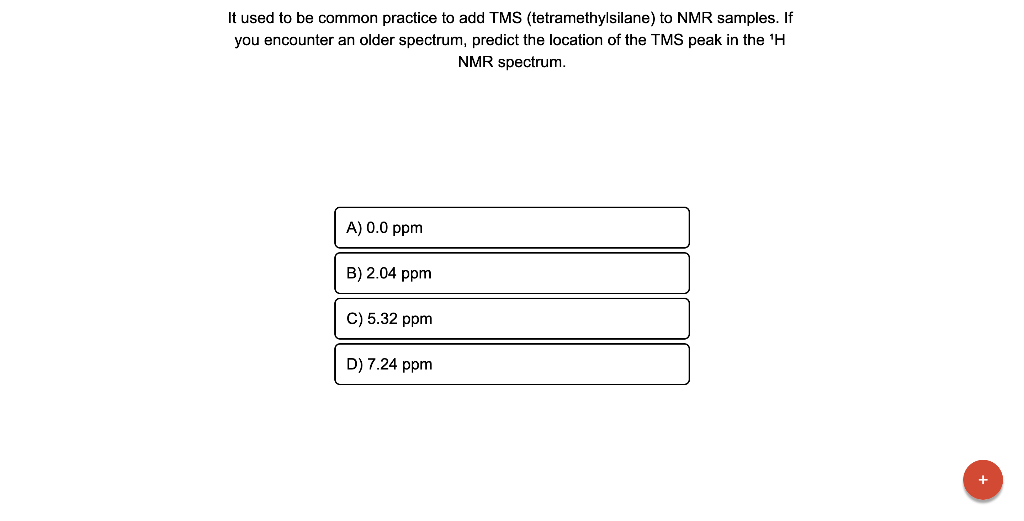
It used to be common practice to add TMS (tetramethylsilane) to NMR samples. If you encounter an older spectrum, predict the Iocation of the TMS peak in the \( { }^{1} \mathrm{H} \) NMR spectrum.
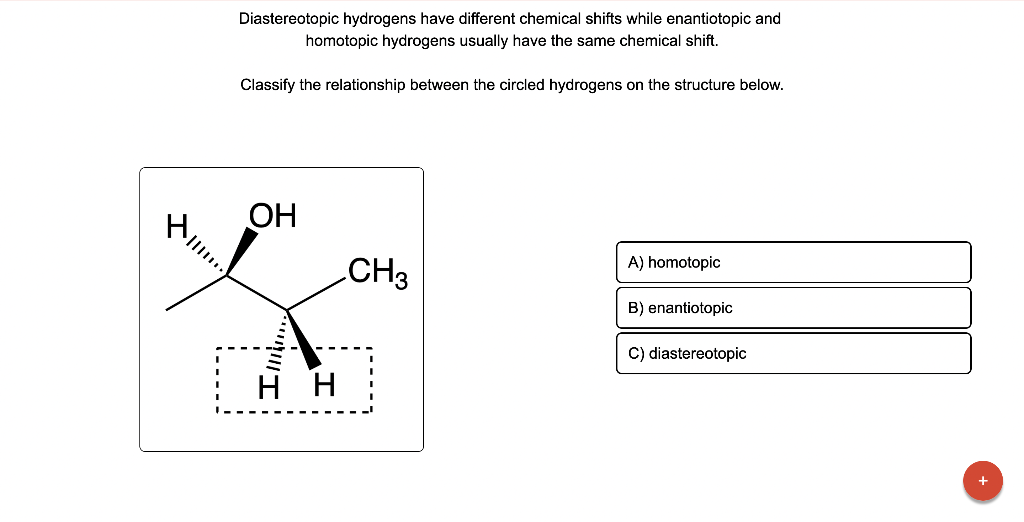
Diastereotopic hydrogens have different chemical shifts while enantiotopic and homotopic hydrogens usually have the same chemical shift. Classify the relationship between the circled hydrogens on the structure below.
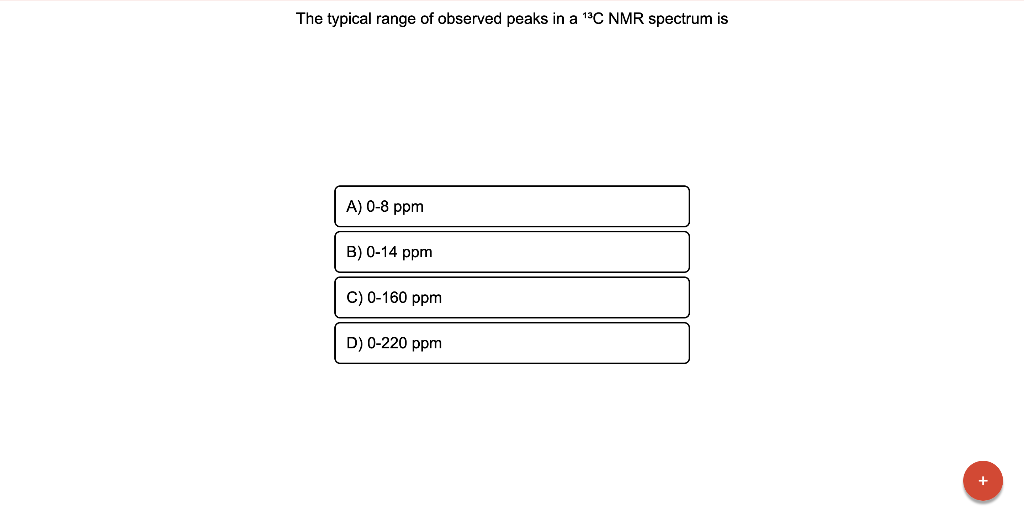
The typical range of observed peaks in a \( { }^{13} \mathrm{C} \) NMR spectrum is
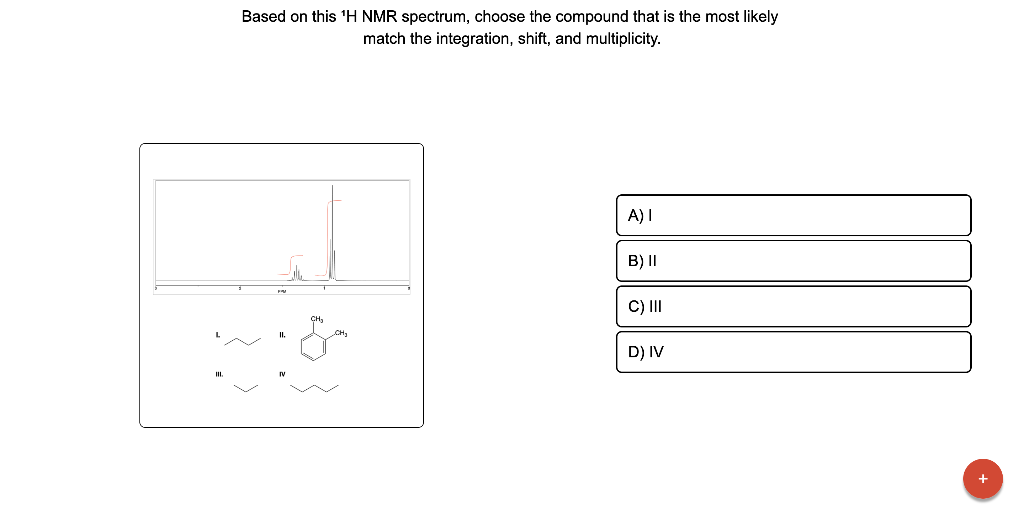
Based on this \( { }^{1} \mathrm{H} \) NMR spectrum, choose the compound that is the most likely match the integration, shift, and multiplicity.
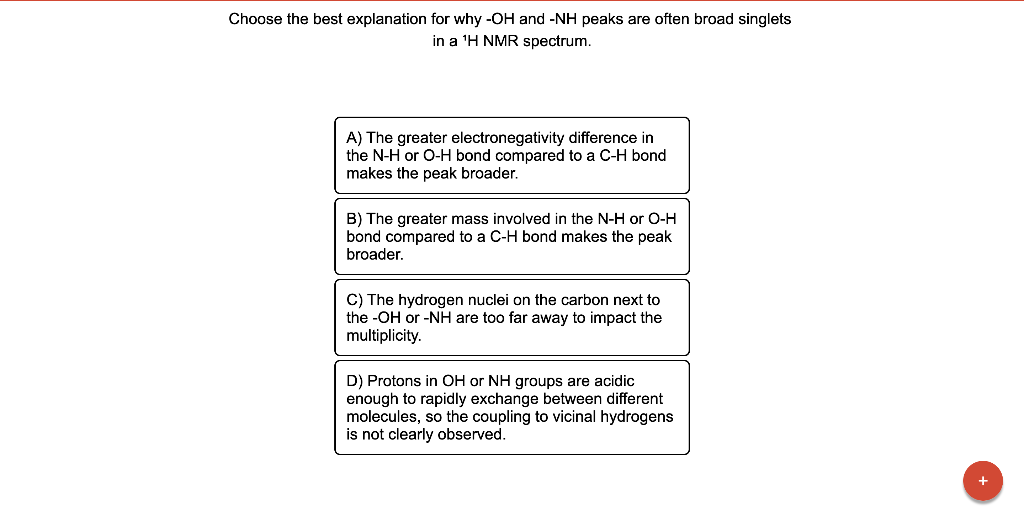
Choose the best explanation for why \( -\mathrm{OH} \) and \( -\mathrm{NH} \) peaks are often broad singlets in a \( { }^{1} \mathrm{H} \) NMR spectrum. A) The greater electronegativity difference in the \( \mathrm{N}-\mathrm{H} \) or \( \mathrm{O}-\mathrm{H} \) bond compared to a \( \mathrm{C}-\mathrm{H} \) bond makes the peak broader. B) The greater mass involved in the \( \mathrm{N}-\mathrm{H} \) or \( \mathrm{O}-\mathrm{H} \) bond compared to a \( \mathrm{C}-\mathrm{H} \) bond makes the peak broader. C) The hydrogen nuclei on the carbon next to the \( -\mathrm{OH} \) or \( -\mathrm{NH} \) are too far away to impact the multiplicity. D) Protons in \( \mathrm{OH} \) or \( \mathrm{NH} \) groups are acidic enough to rapidly exchange between different molecules, so the coupling to vicinal hydrogens is not clearly observed.
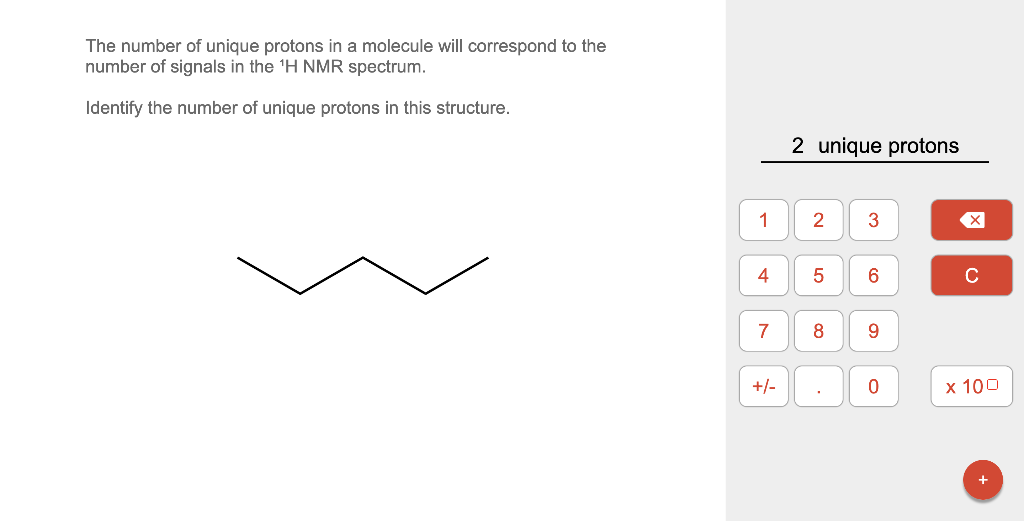
The number of unique protons in a molecule will correspond to the number of signals in the \( { }^{1} \mathrm{H} \) NMR spectrum. Identify the number of unique protons in this structure.
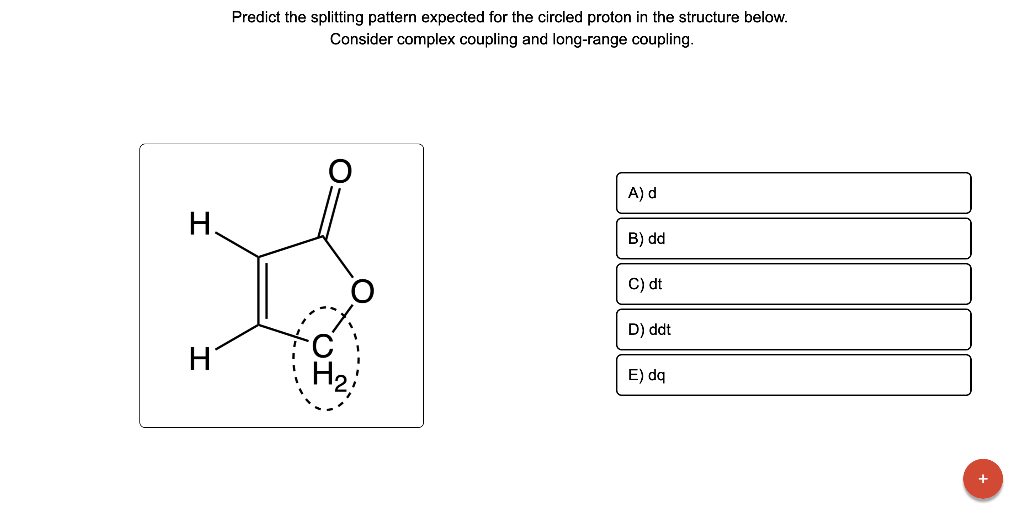
Predict the splitting pattern expected for the circled proton in the structure below. Consider complex coupling and long-range coupling.
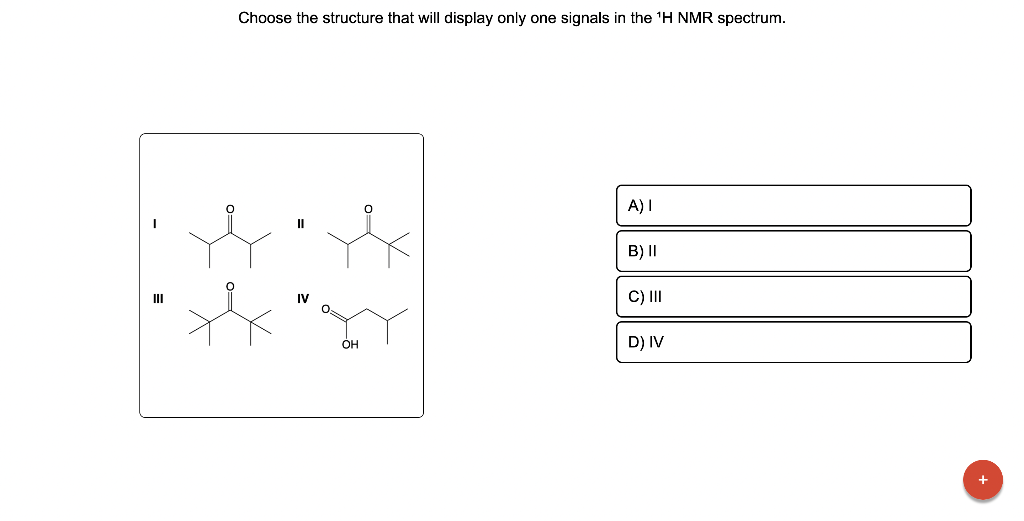
Choose the structure that will display only one signals in the \( { }^{1} \mathrm{H} \) NMR spectrum. I II III IV
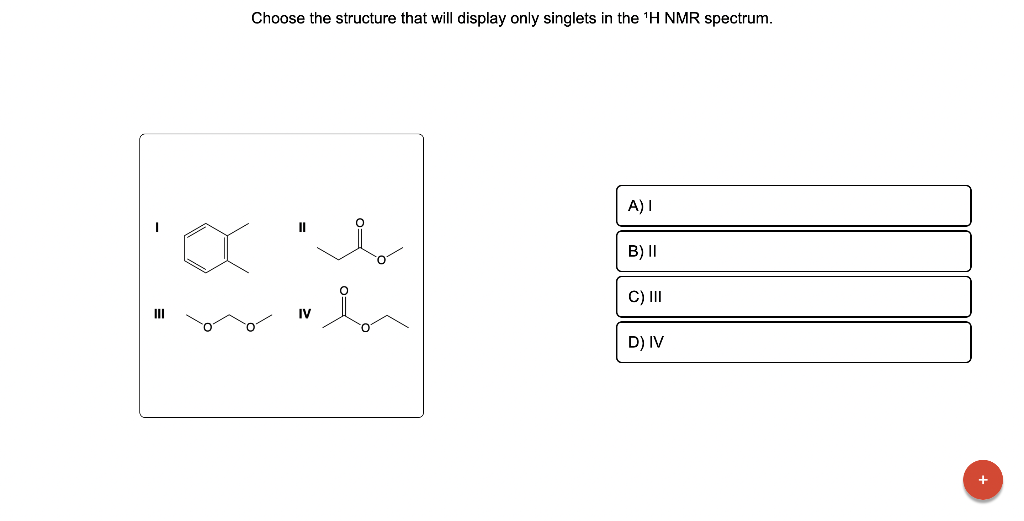
Choose the structure that will display only singlets in the \( { }^{1} \mathrm{H} \) NMR spectrum. I II III IV