Home /
Expert Answers /
Chemistry /
nbsp-nbsp-write-the-cell-notation-for-an-electrochemical-cell-consisting-of-an-anode-where-pa131
(Solved): Write the cell notation for an electrochemical cell consisting of an anode where ...
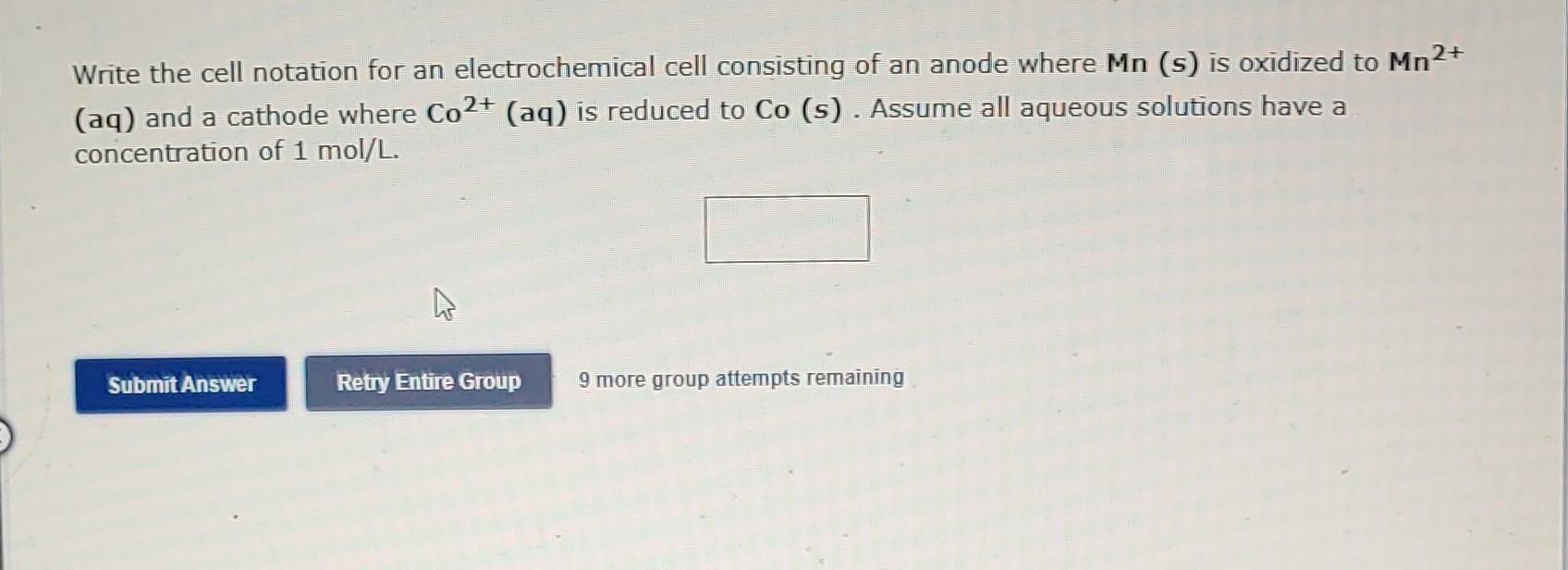

Write the cell notation for an electrochemical cell consisting of an anode where Mn (s) is oxidized to Mn \( { }^{2+} \) (aq) and a cathode where \( \mathrm{Co}^{2+}(\mathrm{aq}) \) is reduced to Co (s). Assume all aqueous solutions have a concentration of \( 1 \mathrm{~mol} / \mathrm{L} \). 9 more group attempts remaining
Write the cell notation for an electrochemical cell consisting of an anode where \( \mathbf{A l}(\mathbf{s}) \) is oxidized to \( \mathbf{A l}^{3+} \) \( \mathbf{( a q )} \) and a cathode where \( \mathbf{F e}^{3+}(\mathbf{a q}) \) is reduced to \( \mathbf{F e}^{2+}(\mathbf{a q}) \) at a platinum electrode. Assume all aqueous solutions have a concentration of \( 1 \mathrm{~mol} / \mathrm{L} \).
Expert Answer
Q-1 Answer of the question is = Mn|Mn2+(1M) || Co2+(1M)|Co Solution :- In the question given, Concentration of [Mn2+] = 1 M Concentration of [Co2+] = 1 M The cathode reaction of the cell is(reduction) -> Co2+(aq) +2e- ? Co(s) ---------(i) The anode r