Home /
Expert Answers /
Chemistry /
nbsp-nbsp-b-on-the-galvanic-cell-label-the-anode-cathode-and-salt-bridge-show-the-direc-pa794
(Solved): b) On the galvanic cell, label the anode, cathode and salt bridge. Show the direc ...
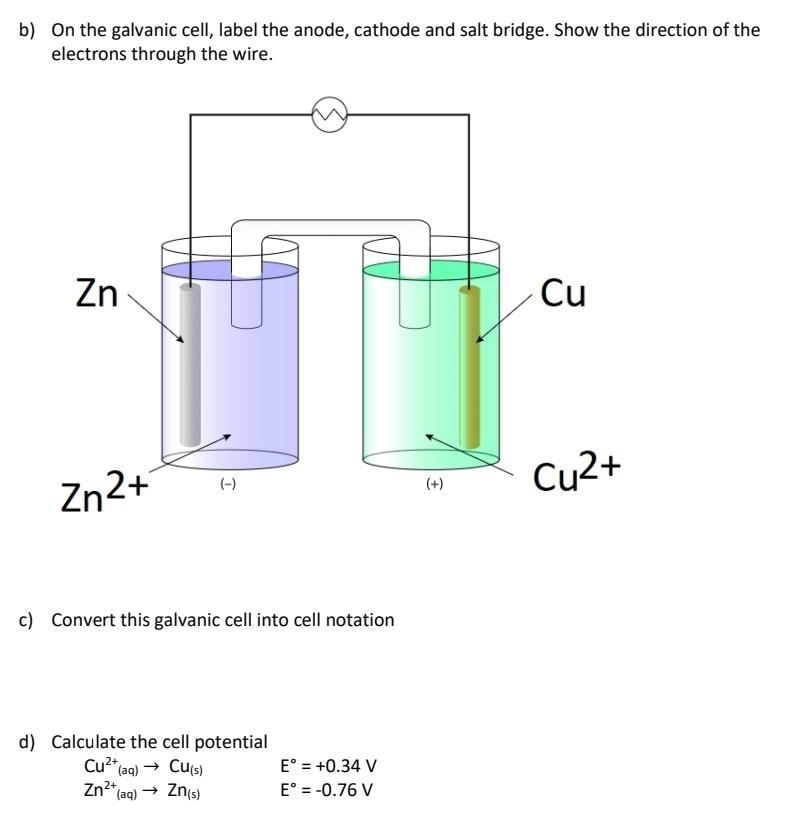
b) On the galvanic cell, label the anode, cathode and salt bridge. Show the direction of the electrons through the wire. c) Convert this galvanic cell into cell notation d) Calculate the cell potential \[ \begin{aligned} \mathrm{Cu}^{2+}{ }_{(\mathrm{aq})} & \rightarrow \mathrm{Cu}_{(\mathrm{s})} & \mathrm{E}^{\circ}=+0.34 \mathrm{~V} \\ \mathrm{Zn}^{2+}{ }_{(\mathrm{aq})} \rightarrow \mathrm{Zn}_{(\mathrm{s})} & \mathrm{E}^{\circ}=-0.76 \mathrm{~V} \end{aligned} \]
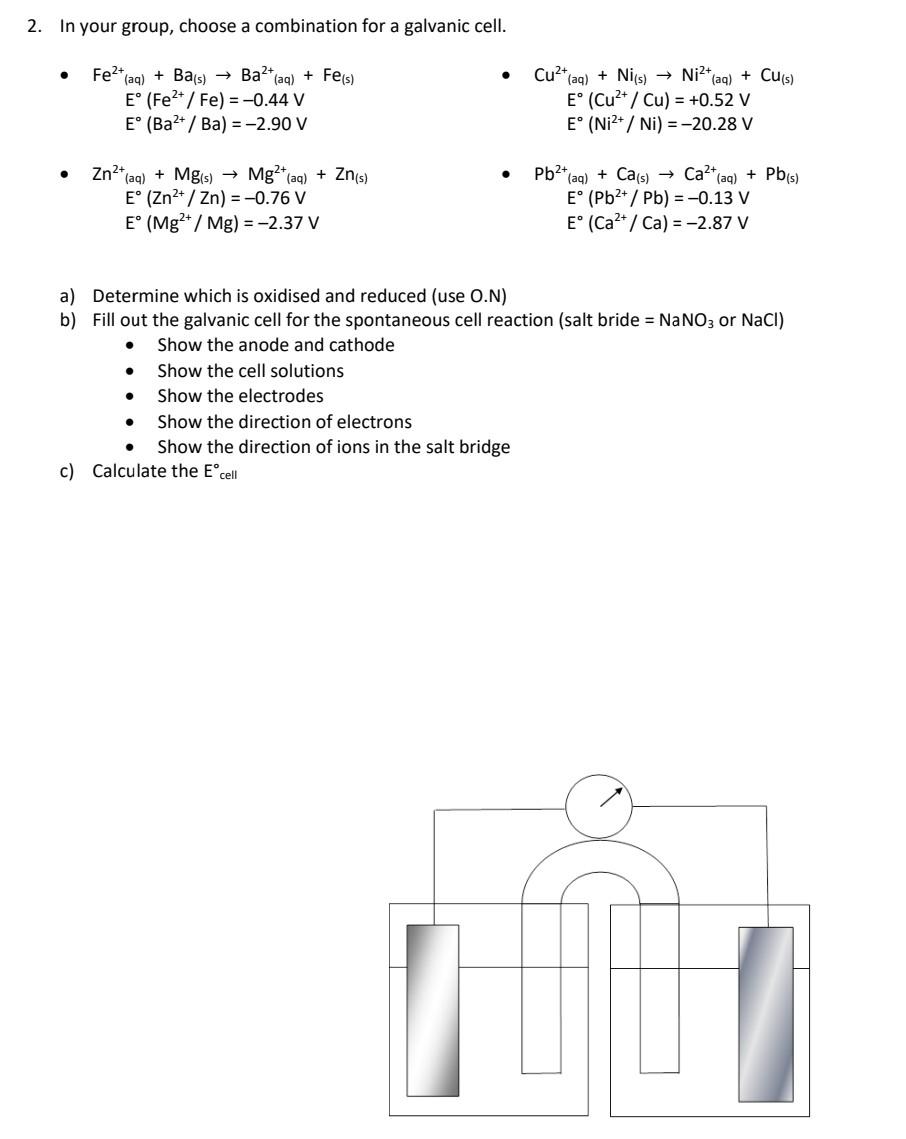
In your group, choose a combination for a galvanic cell. - \( \mathrm{Fe}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Ba}_{(\mathrm{s})} \rightarrow \mathrm{Ba}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Fe}_{(\mathrm{s})} \) - \( \mathrm{Cu}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Ni}_{(\mathrm{s})} \rightarrow \mathrm{Ni}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Cu}_{(\mathrm{s})} \) \( \mathrm{E}^{\circ}\left(\mathrm{Cu}^{2+} / \mathrm{Cu}\right)=+0.52 \mathrm{~V} \) \( \mathrm{E}^{\circ}\left(\mathrm{Ni}^{2+} / \mathrm{Ni}\right)=-20.28 \mathrm{~V} \) - \( \begin{aligned} \mathrm{Zn}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Mg}_{(\mathrm{s})} & \rightarrow \mathrm{Mg}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Zn}_{(\mathrm{s})} \\ \mathrm{E}^{\circ}\left(\mathrm{Zn}^{2+} / \mathrm{Zn}\right)=-0.76 \mathrm{~V} \\ \mathrm{E}^{\circ}\left(\mathrm{Mg}^{2+} / \mathrm{Mg}\right)=-2.37 \mathrm{~V} \end{aligned} \) - \( \begin{aligned} \mathrm{Pb}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Ca}_{(\mathrm{s})} \rightarrow \mathrm{Ca}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Pb}_{(\mathrm{s})} \\ \mathrm{E}^{\circ}\left(\mathrm{Pb}^{2+} / \mathrm{Pb}\right) &=-0.13 \mathrm{~V} \\ \mathrm{E}^{\circ}\left(\mathrm{Ca}^{2+} / \mathrm{Ca}\right) &=-2.87 \mathrm{~V} \end{aligned} \) a) Determine which is oxidised and reduced (use O.N) b) Fill out the galvanic cell for the spontaneous cell reaction (salt bride \( =\mathrm{NaNO}_{3} \) or \( \mathrm{NaCl} \) ) - Show the anode and cathode - Show the cell solutions - Show the electrodes - Show the direction of electrons - Show the direction of ions in the salt bridge c) Calculate the \( E_{\text {cell }}^{\circ} \)