Home /
Expert Answers /
Chemistry /
nbsp-13-based-on-the-dissociation-reaction-given-below-hocl-aq-ho-1-h30-aq-oci-a-pa826
(Solved): 13) Based on the dissociation reaction given below: HOCl(aq) + HO(1) H30* (aq) + OCI (a ...
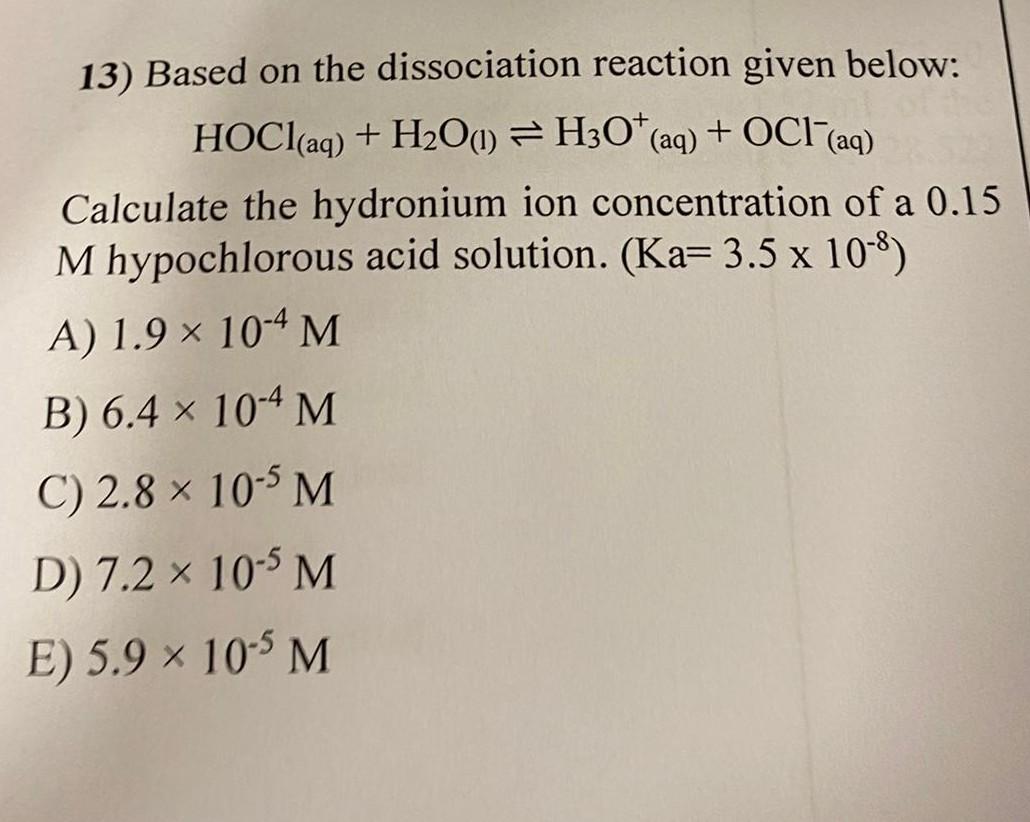
13) Based on the dissociation reaction given below: HOCl(aq) + H?O(1) H30* (aq) + OCI (aq) ? Calculate the hydronium ion concentration of a 0.15 M hypochlorous acid solution. (Ka= 3.5 x 10-8) A) 1.9 × 10-4 M B) 6.4 × 10-4 M C) 2.8 x 10-5 M D) 7.2 × 10-5 M E) 5.9 × 10-5 M