Home /
Expert Answers /
Chemical Engineering /
nbsp-1-the-following-reaction-reaches-equilibrium-at-500c-and-2-bar-4hcl-g-o2-g-2h2o-pa325
(Solved): 1 The following reaction reaches equilibrium at 500C and 2 bar: 4HCl(g) + O2(g) 2H2O ...
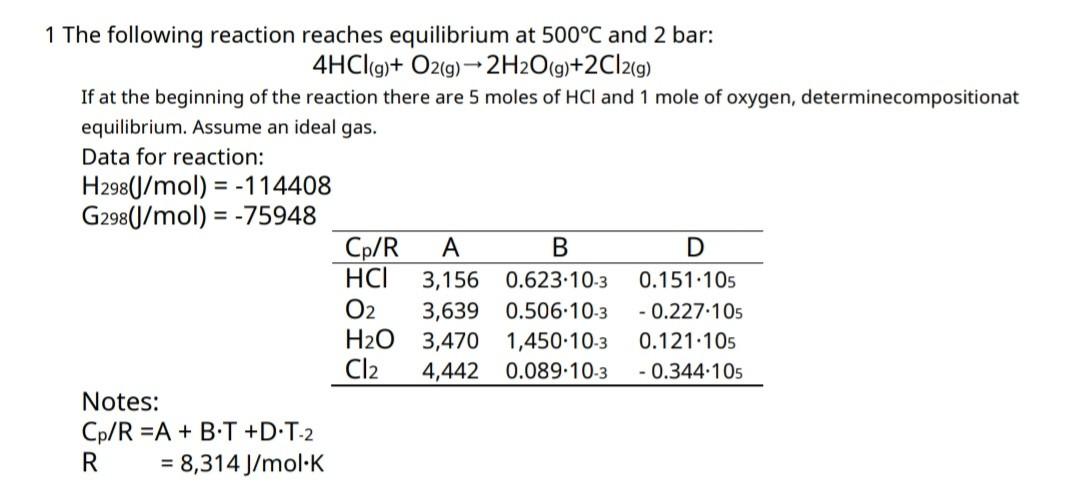
1 The following reaction reaches equilibrium at 500°C and 2 bar: 4HCl(g) + O2(g) ? 2H2O(g)+2Cl2(g) If at the beginning of the reaction there are 5 moles of HCI and 1 mole of oxygen, determinecompositionat equilibrium. Assume an ideal gas. Data for reaction: H298(J/mol) = -114408 G298(J/mol) = -75948 B D Cp/R A HCI 3,156 0.623.10-3 0.151.105 O2 3,639 0.506-10-3 -0.227.105 H?O 3,470 1,450-10-3 0.121.105 Cl2 4,442 0.089-10-3 - 0.344.105 Notes: Cp/R =A + B•T +D:T-2 R = 8,314 J/mol K
Expert Answer
Compound Moles Initially Moles Finally HCl 5 5-4e 02 1 1-e H50 0 2e CU 0 2e e is the extent of reaction Make a separate script and copy paste the given code and run it close all;clc;clear all; %Calculation of Equilibrium constant at 298K %deltaG=-RT*