Home /
Expert Answers /
Chemistry /
missed-this-read-section-20-6-page-an-electrochemical-cell-is-based-on-the-following-two-halfr-pa175
(Solved): MISSED THIS? Read Section 20.6 (Page). An electrochemical cell is based on the following two halfr ...
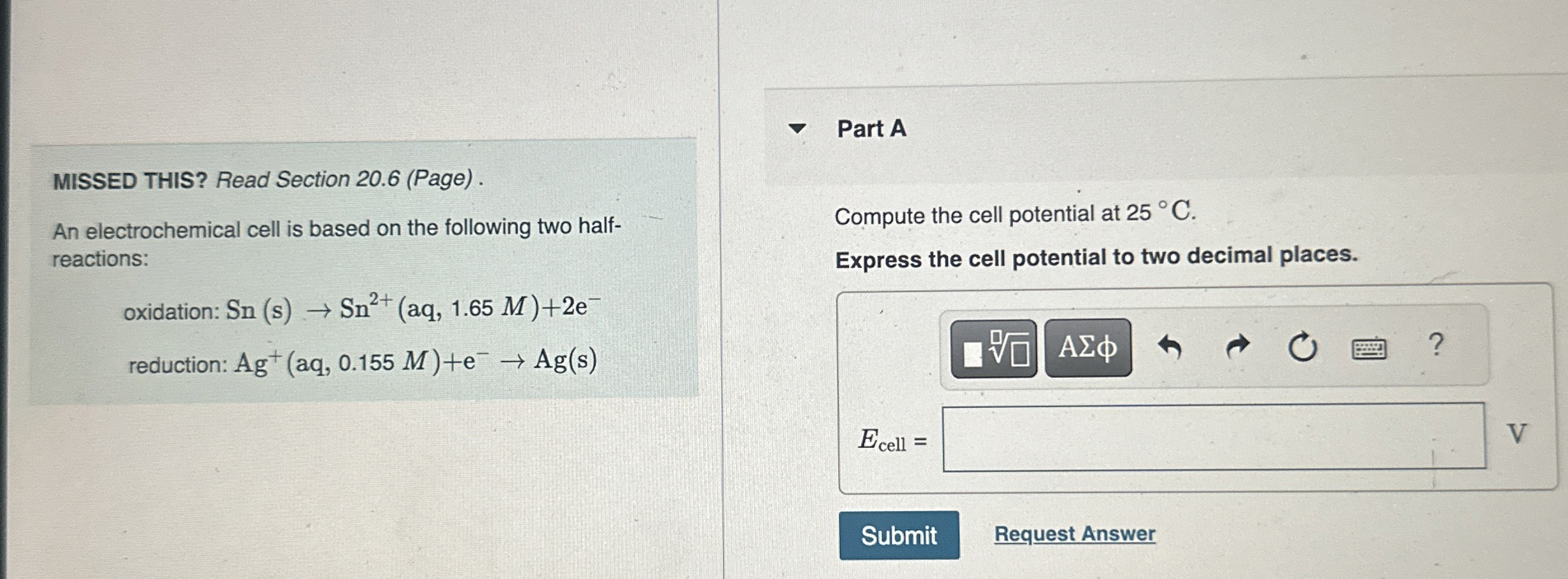
MISSED THIS? Read Section 20.6 (Page). An electrochemical cell is based on the following two halfreactions:
oxidation: Sn(s)->Sn^(2+)(aq,1.65M)+2e^(-)
reduction: Ag^(+)(aq,0.155M)+e^(-)->Ag(s)Part A Compute the cell potential at
25\deg C. Express the cell potential to two decimal places.
◻
E_(cell )=
◻