Home /
Expert Answers /
Chemistry /
labelling-a-galvanic-cell-the-diagram-below-represents-a-daniell-galvanic-cell-involving-zinc-and-pa582
(Solved): Labelling a Galvanic Cell The diagram below represents a Daniell galvanic cell involving zinc and ...
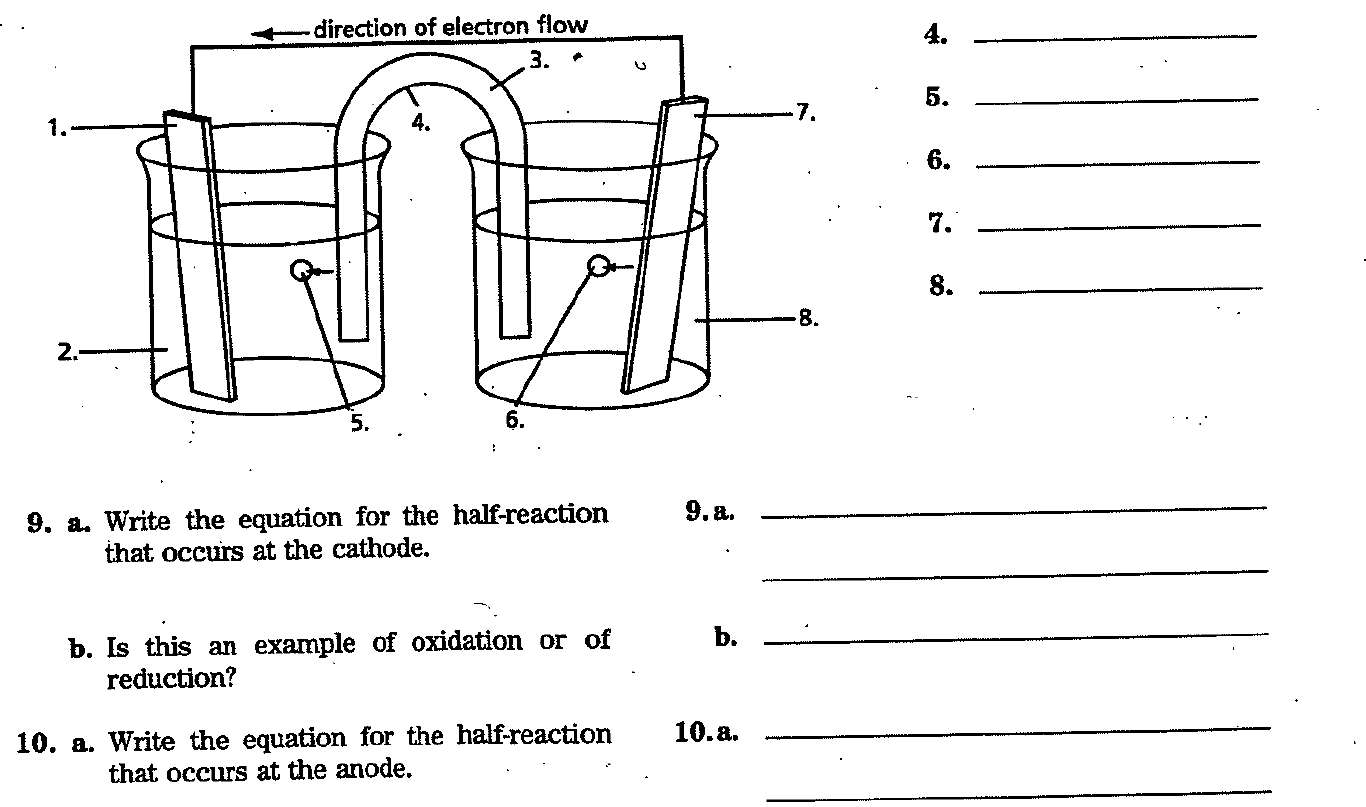
Labelling a Galvanic Cell The diagram below represents a Daniell galvanic cell involving zinc and copper. Match each numbered item in the diagram with the letter of the correct label from the list below. Write your answers in the space provided. E. anode A. Zn²+ solution B. salt bridge C. cathode F. U-tube G. copper cation D. zinc cation H. Cut solution 1. 2. 3.
direction of electron flow 3. * 2. 6. 9. a. Write the equation for the half-reaction that occurs at the cathode. b. Is this an example of oxidation or of reduction? 10. a. Write the equation for the half-reaction that occurs at the anode. 1. U 9.a. b. 10.a. -7. 8. 4. 5. 6. 7. 8.
b. Is this an example of oxidation or of reduction? 11. Write the overall ionic equation. ¹2. Write the overall molecular equation. b. 11. 12.
Expert Answer
Answer The direction of flow of electrons is from anode to ca