Home /
Expert Answers /
Chemistry /
just-need-the-first-sheet-filled-out-the-rest-just-posted-in-case-you-need-nbsp-a-separate-page-pa470
(Solved): just need the first sheet filled out. the rest just posted in case you need :) A separate page ...
just need the first sheet filled out. the rest just posted in case you need :)
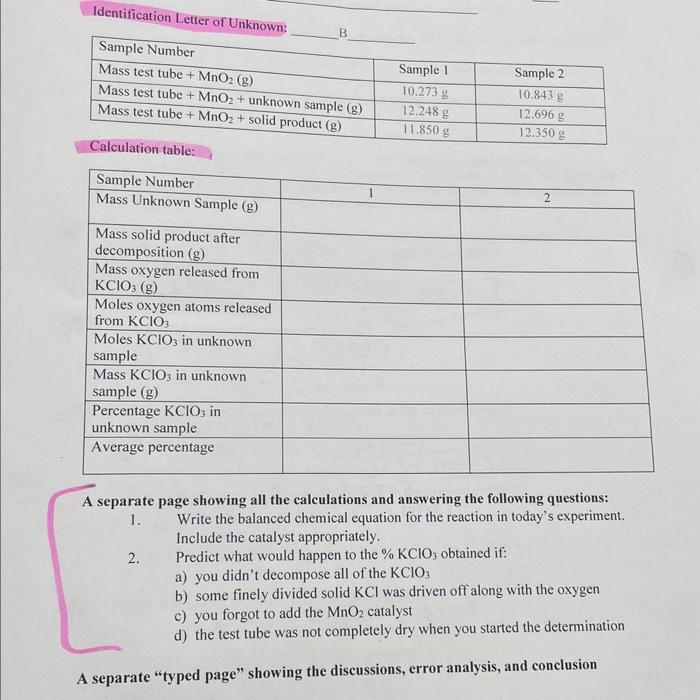








A separate page showing all the calculations and answering the following questions: 1. Write the balanced chemical equation for the reaction in today's experiment. Include the catalyst appropriately. 2. Predict what would happen to the \( \% \mathrm{KClO}_{3} \) obtained if: a) you didn't decompose all of the \( \mathrm{KClO}_{3} \) b) some finely divided solid \( \mathrm{KCl} \) was driven off along with the oxygen c) you forgot to add the \( \mathrm{MnO}_{2} \) catalyst d) the test tube was not completely dry when you started the determination A senarate "typed page" showing the discussions, error analysis, and conclusion
A student heats a \( 2.8594 \) gram sample of a mixture that contains an unknown amount of potassium chlorate \( \left(\mathrm{KClO}_{3}\right) \) in a test tube in the presence of some \( \mathrm{MnO}_{2} \) catalyst. The \( \mathrm{KClO}_{3} \) in the mixture decomposes during the heating process, releasing its oxygen. After the sample has cooled the student finds the mass of the sample residue to be \( 2.1203 \) grams. 1. How many grams of oxygen atoms were in the \( \mathrm{KClO}_{3} \) in the original sample? bafore heating \( =2,85949 \) (before-after) after heating \( =2.12039 \mathrm{~g} \) (before-after) \( (2.85949)-(2.12039) \) 2. How many moles of oxygen atoms is this? \[ \begin{aligned} (0.739) 9) \div(32,09 \mathrm{~g} \text { mol }\\ &=0.0231 \mathrm{~mol} \end{aligned} \] 3. How many moles of \( \mathrm{KClO}_{3} \) must have been in the original sample? \[ \begin{aligned} =&(0.0231 \mathrm{~mol}) \times(2 / 3) \\ =&(0.0231 \mathrm{~mol}) \times(0.667) \\ &=0.0154 \mathrm{~mol} \end{aligned} \] How many grams of \( \mathrm{KClO}_{3} \) must have been in the original sample? \( =(0.054 \mathrm{~mol}) \times(122.55 \mathrm{~g} / \mathrm{mol}) \) \[ =1.8870 \mathrm{~g} \] What is the \( \% \) of \( \mathrm{KClO}_{3} \) in the original sample?
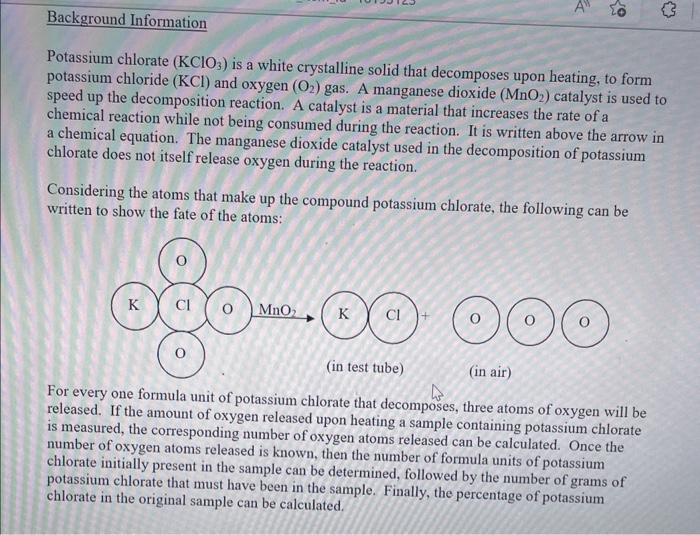
Potassium chlorate \( \left(\mathrm{KClO}_{3}\right) \) is a white crystalline solid that decomposes upon heating, to form potassium chloride \( (\mathrm{KCl}) \) and oxygen \( \left(\mathrm{O}_{2}\right) \) gas. A manganese dioxide \( \left(\mathrm{MnO}_{2}\right) \) catalyst is used to speed up the decomposition reaction. A catalyst is a material that increases the rate of a chemical reaction while not being consumed during the reaction. It is written above the arrow in a chemical equation. The manganese dioxide catalyst used in the decomposition of potassium chlorate does not itself release oxygen during the reaction. Considering the atoms that make up the compound potassium chlorate, the following can be written to show the fate of the atoms: For every one formula unit of potassium chlorate that decomposes, three atoms of oxygen will be released. If the amount of oxygen released upon heating a sample containing potassium chlorate is measured, the corresponding number of oxygen atoms released can be calculated. Once the number of oxygen atoms released is known, then the number of formula units of potassium chlorate initially present in the sample can be determined, followed by the number of grams of potassium chlorate that must have been in the sample. Finally, the percentage of potassium chlorate in the original sample can be calculated.
Add a small spatula (not a heaping, spatula) of manganese dioxide to a clean, dry PYREX test tube. Determine the mass of the test tube and \( \mathrm{MnO}_{2} \) on the analytical balance. Experience has shown that more stable mass readings are obtained when the test tube is placed upright on the balance pan rather than lying horizontal. A \( 125 \mathrm{~mL} \) Erlenmeyer flask is used to hold the test tube vertical. Place the flask on the balance and tare (reset the zero). Then add the test tube with \( \mathrm{MnO}_{2} \) to the flask. The mass displayed will be just the mass of the test tube and \( \mathrm{MnO}_{2} \). Record the mass of the test tube and \( \mathrm{MnO}_{2} \) in the data table in your report sheet. Select one of the unknown mixtures and record its identity in your journal. Add approximately \( 1.5-2.0 \mathrm{~g} \) of the unknown mixture to the test tube containing \( \mathrm{MnO}_{2} \) and measure and record its mass as described above. Mix the unknown sample with the \( \mathrm{MnO}_{2} \) by carefully rolling the test tube on the laboratory bench until the contents appear gray. Place a 3-pronged, or similar clamp on a ring stand and attach the test tube in the clamp near its mouth. Adjust the clamp so that the test tube is nearly horizontal. Then tap it gently to spread out the mixture, making plenty of air space above the sample. -Hold a laboratory burner with a low blue flame by its base and very gently heat the test tube along the length of the mixture until it melts and begins to froth. Take care to keep the mixture from frothing into the top half of the test tube. Remove the heat as needed. Be very careful not to heat or burn the test tube clamp. CAUTION Before proceeding be sure the opening to the test tube is not blocked. If it is, consult your instructor.
After the bubbling stops, heat the mixture to a "red hot" condition. Apply the lab burner to the material closest to the mouth of the test tube until it appears red. (The mixture in the test tube may look like a red glowing ember.) If more bubbling occurs, back off with the heat-to prevent vigorous frothing. Once this section of the mixture has been red hot, continue heating the rest of the mixture by moving the burner along the test tube toward the bottom of the tube until all of the mixture has been red hot and no more bubbling is observed. Occasionally a white smoke can be seen coming out of test tube when it is heated very strongly. If this happens, remove the lab burner, let the tube cool for a minute, and then reapply the heat. After all of the mixture remaining in the test tube has been heated to red hot, cool the tube to room temperature. Determine the mass of the test tube, \( \mathrm{MnO}_{2} \), and remaining product as before and record the data in your report sheet. If the tube is not completely cooled, your mass will be in error. If the balance reading is not stable, your tube may still be warm. If you are unsure if your sample was heated to completion, you can reheat the sample and mass again. The masses should be consistent if the reaction has completed. Better experimental results are obtained when procedures are repeated and results averaged. This is especially important when working with solid mixtur? that are not homogeneous and one sample may not be representative of the whole. Repeat the decomposition procedure using a second sample of the same unknown. Record the data from the second sample in your data table next to that from the first sample. Waste Disposal: Put the material remaining in the test tubes into the labeled container provided. Thourally wash the used test tubes with soap and water.