Home /
Expert Answers /
Chemistry /
j-what-is-the-hybridization-of-the-central-carbon-of-1-2-propadiene-an-allene-i-mathrm-sp-pa833
(Solved): j. What is the hybridization of the central carbon of 1,2-propadiene (an allene): i. \( \mathrm{sp ...
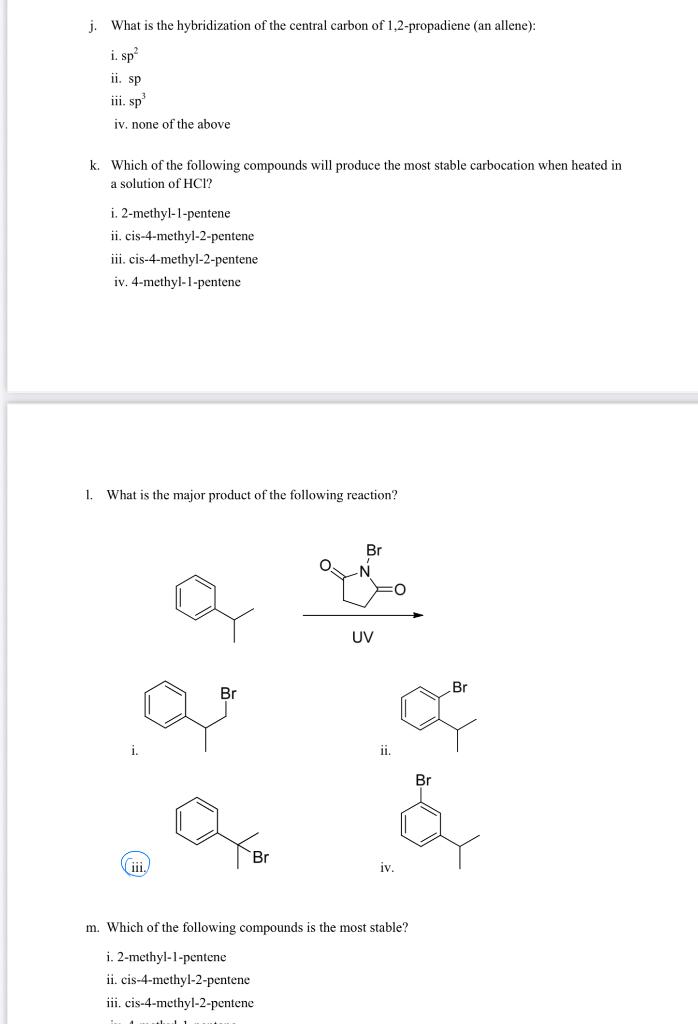
j. What is the hybridization of the central carbon of 1,2-propadiene (an allene): i. \( \mathrm{sp}^{2} \) ii. \( s p \) iii. \( \mathrm{sp}^{3} \) iv. none of the above k. Which of the following compounds will produce the most stable carbocation when heated in a solution of \( \mathrm{HCl} \) ? i. 2-methyl-1-pentene ii. cis-4-methyl-2-pentene iii. cis-4-methyl-2-pentene iv. 4-methyl-1-pentene 1. What is the major product of the following reaction? i. ii. (iii.) iv. \( \mathrm{m} \). Which of the following compounds is the most stable? i. 2-methyl-1-pentene ii. cis-4-methyl-2-pentene iii. cis-4-methyl-2-pentene