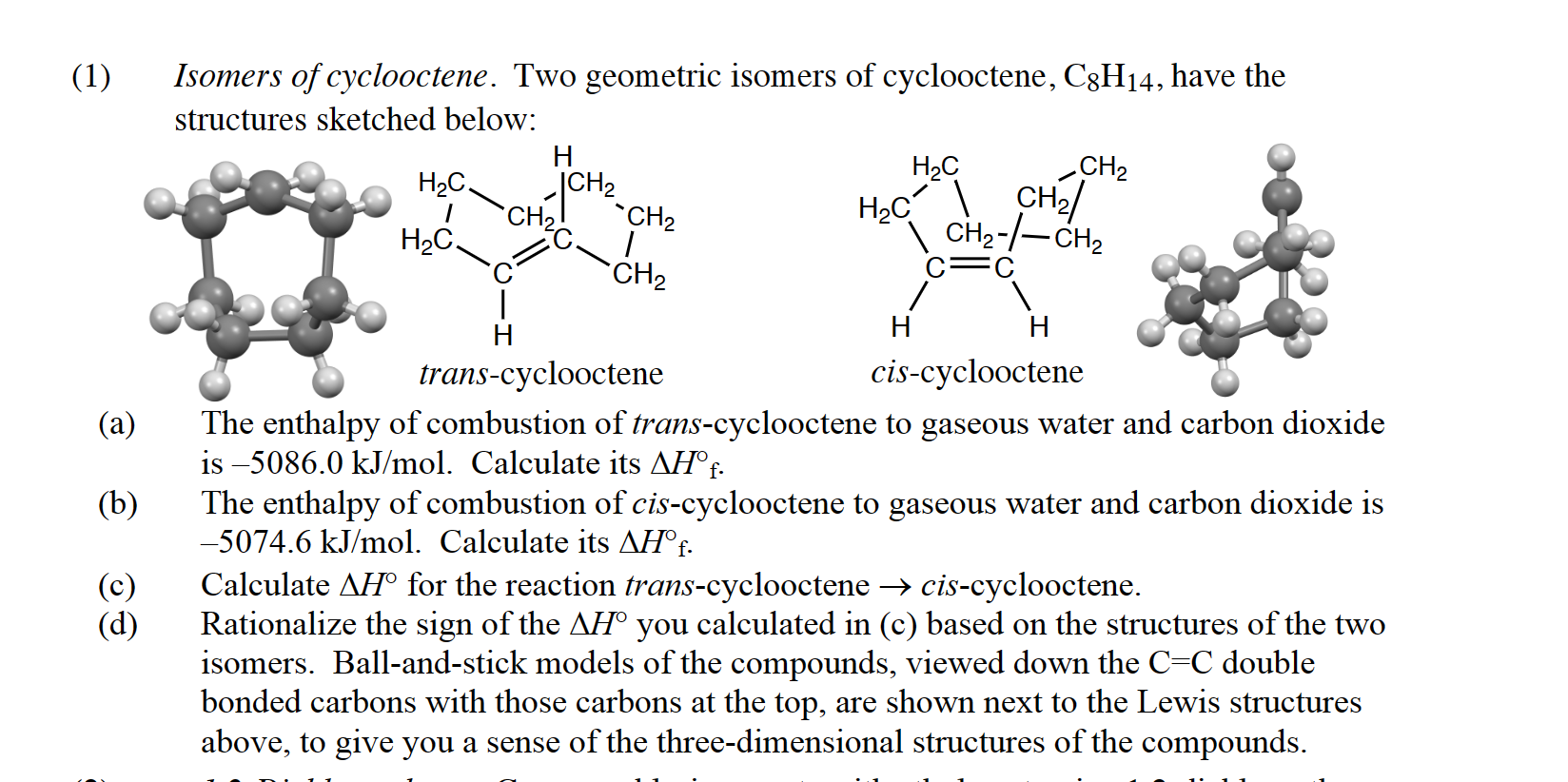
(Solved): Isomers of cyclooctene. Two geometric isomers of cyclooctene, C 8 H 14 , have the structures sketche ...
Isomers of cyclooctene. Two geometric isomers of cyclooctene, C 8 H 14 , have the structures sketched below: (a) The enthalpy of combustion of trans-cyclooctene to gaseous water and carbon dioxide is –5086.0 kJ/mol. Calculate its ∆H°f . (b) The enthalpy of combustion of cis-cyclooctene to gaseous water and carbon dioxide is –5074.6 kJ/mol. Calculate its ∆H°f . (c) Calculate ∆H° for the reaction trans-cyclooctene ® cis-cyclooctene. (d) Rationalize the sign of the ∆H° you calculated in (c) based on the structures of the two isomers. Ball-and-stick models of the compounds, viewed down the C=C double bonded carbons with those carbons at the top, are shown next to the Lewis structures above, to give you a sense of the three-dimensional structures of the compounds.