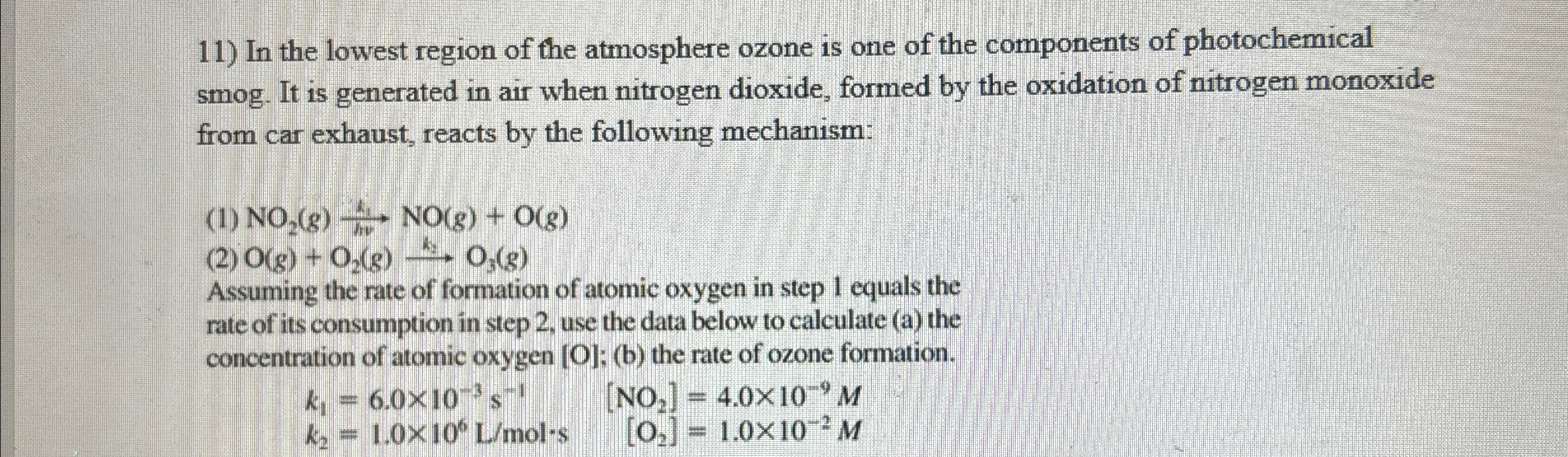Home /
Expert Answers /
Chemistry /
in-the-lowest-region-of-the-atmosphere-ozone-is-one-of-the-components-of-photochemical-smog-it-is-g-pa917
(Solved): In the lowest region of the atmosphere ozone is one of the components of photochemical smog. It is g ...
In the lowest region of the atmosphere ozone is one of the components of photochemical smog. It is generated in air when nitrogen dioxide, formed by the oxidation of nitrogen monoxide from car exhaust, reacts by the following mechanism: (1)
NO_(2)(g)(k_(1))/(h)
u NO(g)+O(g)(2)
O(g)+O_(2)(g)->sk_(4)O_(3)(g)Assuming the rate of formation of atomic oxygen in step 1 equals the rate of its consumption in step 2 , use the data below to calculate (a) the concentration of atomic oxygen [O]; (b) the rate of ozone formation.
k_(1)=6.0\times 10^(-3)s^(-1),[NO_(2)]=4.0\times 10^(-9)M
k_(2)=1.0\times 10^(6)(L)/(m)ol*s,[O_(2)]=1.0\times 10^(-2)M