Home /
Expert Answers /
Chemical Engineering /
in-a-jacketed-continuous-stirred-tank-reactor-the-first-order-exothermic-reaction-a-rightarro-pa843
(Solved): In a jacketed continuous stirred tank reactor, the first-order exothermic reaction \( A \rightarro ...
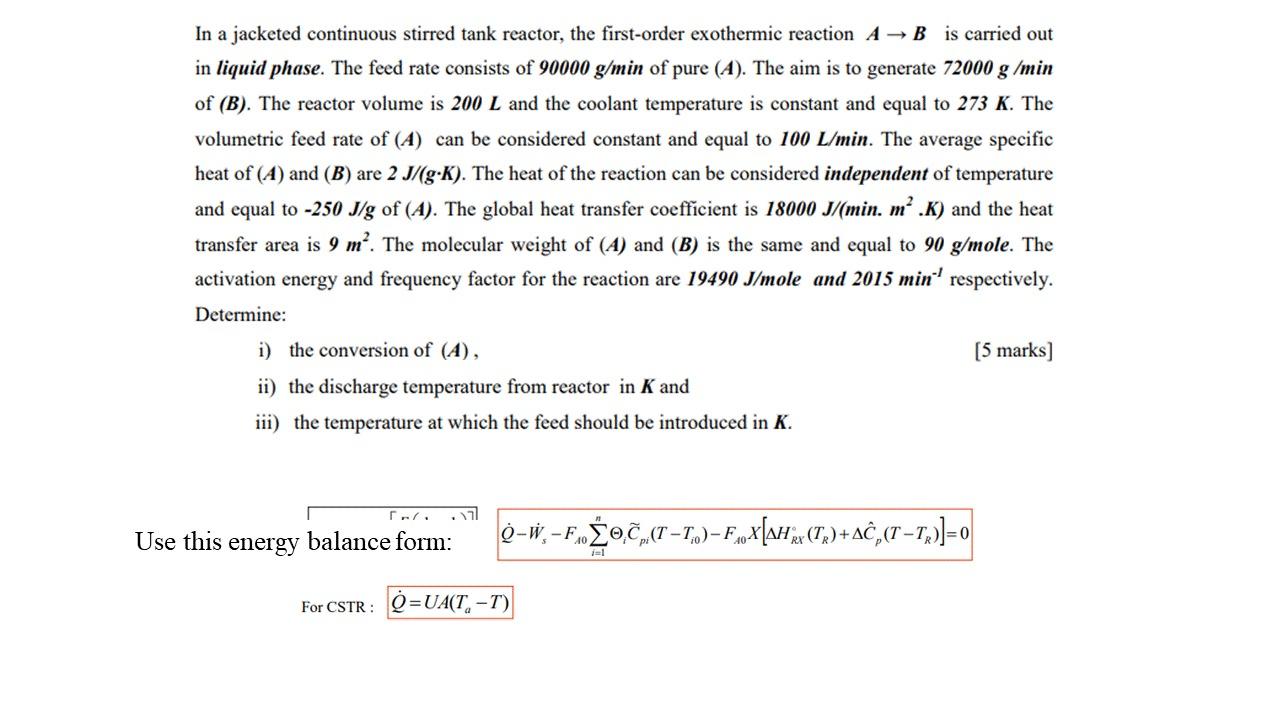
In a jacketed continuous stirred tank reactor, the first-order exothermic reaction \( A \rightarrow B \) is carried out in liquid phase. The feed rate consists of \( 90000 \mathrm{~g} / \mathrm{min} \) of pure \( (A) \). The aim is to generate \( 72000 \mathrm{~g} / \mathrm{min} \) of \( (B) \). The reactor volume is \( 200 L \) and the coolant temperature is constant and equal to \( 273 \mathrm{~K} \). The volumetric feed rate of \( (A) \) can be considered constant and equal to \( 100 \mathrm{~L} / \mathrm{min} \). The average specific heat of \( (A) \) and \( (B) \) are \( 2 J /(g \cdot K) \). The heat of the reaction can be considered independent of temperature and equal to \( -250 \mathrm{~J} / \mathrm{g} \) of \( (A) \). The global heat transfer coefficient is \( 18000 \mathrm{~J} /\left(\min . \mathrm{m}^{2} . K\right) \) and the heat transfer area is \( 9 \mathrm{~m}^{2} \). The molecular weight of \( (A) \) and \( (B) \) is the same and equal to \( 90 \mathrm{~g} / \mathrm{mole} \). The activation energy and frequency factor for the reaction are \( 19490 \mathrm{~J} / \mathrm{mole}^{\text {and }} 2015 \mathrm{~min}^{-1} \) respectively. Determine: i) the conversion of \( (A) \), [5 marks] ii) the discharge temperature from reactor in \( \boldsymbol{K} \) and iii) the temperature at which the feed should be introduced in \( \boldsymbol{K} \). Use this energy balance form: \( \dot{Q}-\dot{W}_{s}-F_{A 0} \sum_{i=1}^{n} \Theta_{i} \widetilde{C}_{p i}\left(T-T_{i 0}\right)-F_{A 0} X\left[\Delta H_{R X}^{\circ}\left(T_{R}\right)+\Delta \hat{C}_{p}\left(T-T_{R}\right)\right]=0 \) For CSTR : \( \quad \dot{Q}=U A\left(T_{a}-T\right) \)
Expert Answer
To determine the conversion of A, the discharge temperature from the reactor, and the temperature at which the feed should be introduced in a jacketed continuous stirred tank reactor, you can use the given information and the energy balance equation