Home /
Expert Answers /
Chemistry /
in-a-coffee-cup-calorimeter-140-0-mathrm-ml-of-1-0-mathrm-m-mathrm-naoh-a-pa990
(Solved): In a coffee-cup calorimeter, \( 140.0 \mathrm{~mL} \) of \( 1.0 \mathrm{M} \) \( \mathrm{NaOH} \) a ...
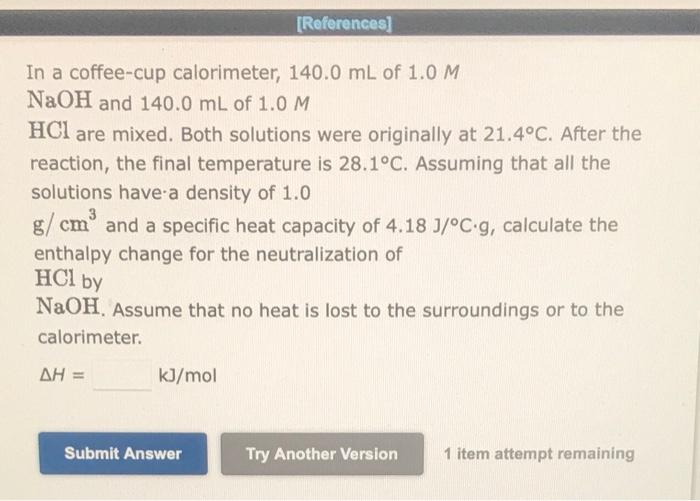
In a coffee-cup calorimeter, \( 140.0 \mathrm{~mL} \) of \( 1.0 \mathrm{M} \) \( \mathrm{NaOH} \) and \( 140.0 \mathrm{~mL} \) of \( 1.0 \mathrm{M} \) \( \mathrm{HCl} \) are mixed. Both solutions were originally at \( 21.4^{\circ} \mathrm{C} \). After the reaction, the final temperature is \( 28.1^{\circ} \mathrm{C} \). Assuming that all the solutions have a density of \( 1.0 \) \( \mathrm{g} / \mathrm{cm}^{3} \) and a specific heat capacity of \( 4.18 \mathrm{~J} /{ }^{\circ} \mathrm{C} \cdot \mathrm{g} \), calculate the enthalpy change for the neutralization of \( \mathrm{HCl} \) by \( \mathrm{NaOH} \). Assume that no heat is lost to the surroundings or to the calorimeter. \[ \Delta H=\quad \mathrm{kJ} / \mathrm{mol} \] 1 item attempt remaining