Home /
Expert Answers /
Chemistry /
im-am-totally-lost-nbsp-in-an-aqueous-chloride-solution-cobalt-ii-exists-in-equilibrium-with-the-c-pa997
(Solved): im am totally lost In an aqueous chloride solution cobalt(II) exists in equilibrium with the c ...
im am totally lost
![\[
\mathrm{CoCl}_{4}^{2-}(a q) \rightleftharpoons \mathrm{Co}^{2+}(a q)+4 \mathrm{Cl}^{-}(a q)
\]
ean conclude that:
1. This](https://media.cheggcdn.com/study/70b/70b16b3c-dd26-47bb-b7b3-c400afec3c22/image)

![\[
\mathrm{CoCl}_{4}^{2-}(a q) \rightleftharpoons \mathrm{Co}^{2+}(a q)+4 \mathrm{Cl}^{-}(a q)
\]
ean conclude that:
1. This](https://media.cheggcdn.com/study/70b/70b16b3c-dd26-47bb-b7b3-c400afec3c22/image)
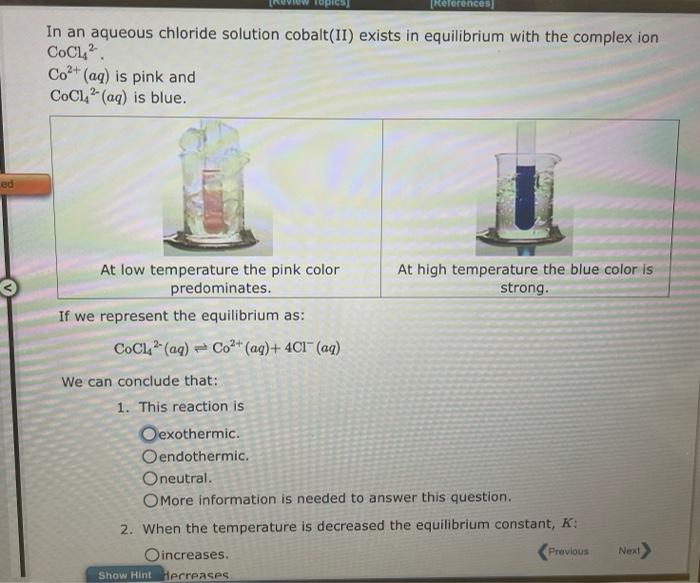
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion \( \mathrm{CoCl}_{4}{ }^{2-} \). \( \mathrm{Co}^{2+}(a q) \) is pink and \( \mathrm{CoCl}_{4}{ }^{2-}(a q) \) is blue. \( \begin{array}{ll}\text { At low ............ predominates. } & \text { At high temperature the blue color is } \\ \text { strong. }\end{array} \) If we represent the equilibrium as: \[ \mathrm{CoCl}_{4}{ }^{2-}(a q) \rightleftharpoons \mathrm{Co}^{2+}(a q)+4 \mathrm{Cl}^{-}(a q) \]
\[ \mathrm{CoCl}_{4}^{2-}(a q) \rightleftharpoons \mathrm{Co}^{2+}(a q)+4 \mathrm{Cl}^{-}(a q) \] ean conclude that: 1. This reaction is exothermic. endothermic. neutral. More information is needed to answer this question. 2. When the temperature is decreased the equilibrium constant, \( K \) : increases. decreases. remains the same. More information is needed to answer this question. 3. When the temperature is decreased the equilibrium concentration of \( \mathrm{CoCl}_{4}{ }^{2 .} \) increases. decreases. remains the same. More information is needed to answer this question. No more group attempts remain
Expert Answer
1. This reaction is exothermi