Home /
Expert Answers /
Chemistry /
ii-use-the-following-data-to-calculate-a-value-for-the-enthalpy-change-of-solution-of-copper-ii-pa292
(Solved): (ii) Use the following data to calculate a value for the enthalpy change of solution of copper(II) ...
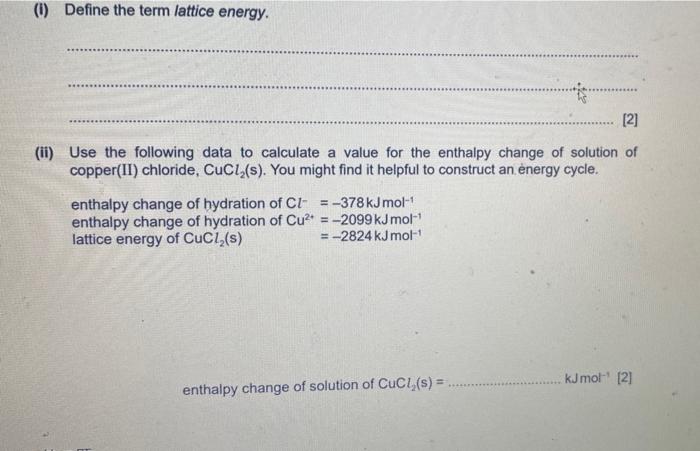
(ii) Use the following data to calculate a value for the enthalpy change of solution of copper(II) chloride, \( \mathrm{CuCl}_{2} \) (s). You might find it helpful to construct an ènergy cycle. enthalpy change of hydration of \( \mathrm{Cl}^{-}=-378 \mathrm{~kJ} \mathrm{~mol}^{-1} \) enthalpy change of hydration of \( \mathrm{Cu}^{2+}=-2099 \mathrm{~kJ} \mathrm{~mol}^{-1} \) lattice energy of \( \mathrm{CuCl}_{2}(\mathrm{~s}) \) \( =-2824 \mathrm{~kJ} \mathrm{~mol}^{-1} \) enthalpy change of solution of \( \mathrm{CuCl}_{2}(\mathrm{~s})= \) \( \mathrm{kJmol}^{-1}[2] \)
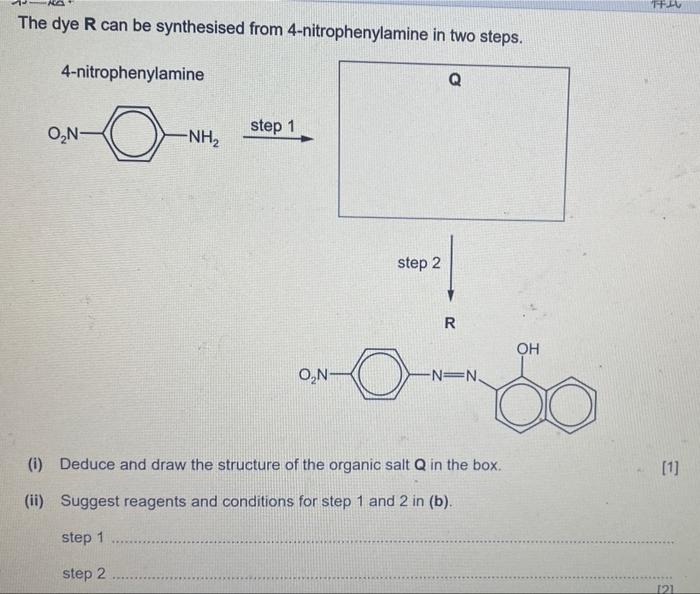
The dye \( \mathbf{R} \) can be synthesised from 4-nitrophenylamine in two steps. step 2 \( \mathbf{R} \) (i) Deduce and draw the structure of the organic salt \( Q \) in the box. [1] (ii) Suggest reagents and conditions for step 1 and 2 in (b). step 1 step 2