Home /
Expert Answers /
Chemistry /
if-no-precipitate-forms-in-the-reaction-enter-nr-no-reaction-after-the-reaction-arrow-reaction-pa415
(Solved): If no precipitate forms in the reaction, enter NR (no reaction) after the reaction arrow. Reaction ...
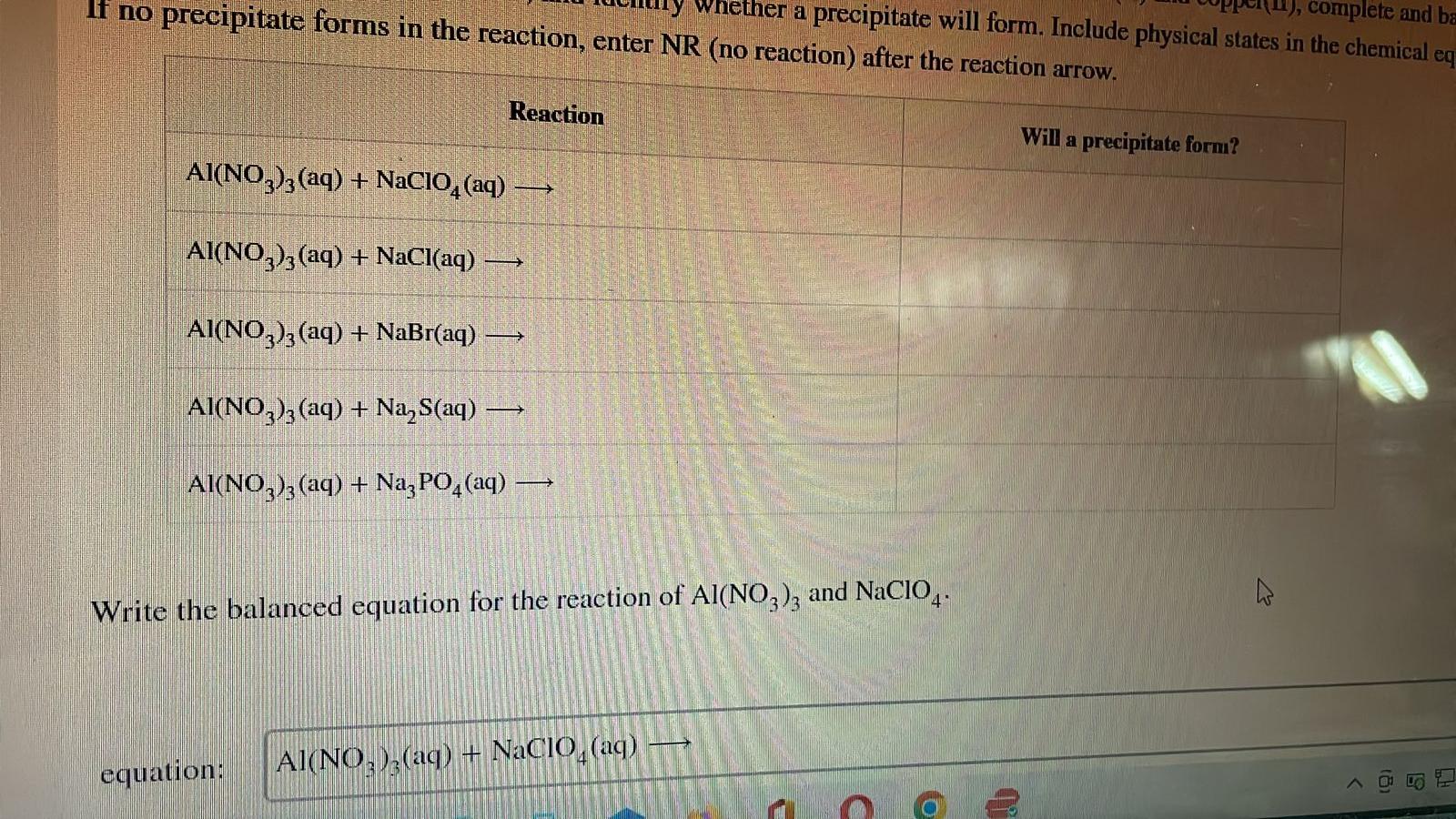
If no precipitate forms in the reaction, enter NR (no reaction) after the reaction arrow. Reaction AI(NO3)3(aq) + NaClO4 (aq) AI(NO3)3(aq) + NaCl(aq) — AI(NO3)3(aq) + NaBr(aq) -> AI(NO3)3(aq) + Na?S(aq) AI(NO3)3 (aq) + Na3PO4 (aq) -> - Whether a precipitate will form. Include physical states in the chemical eq 1), complete and ba equation: Al(NO?)2(aq) + NaClO (aq) Write the balanced equation for the reaction of Al(NO3)3 and NaClO4. 3 42 C (o V Will a precipitate form? 2 < (8) B H