Home /
Expert Answers /
Chemistry /
identify-whether-the-following-compounds-are-expected-to-be-soluble-or-insoluble-based-on-the-solub-pa727
(Solved): Identify whether the following compounds are expected to be soluble or insoluble based on the solub ...
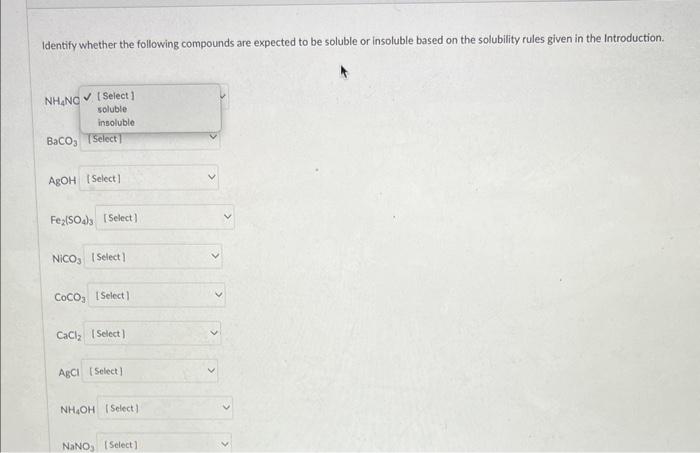
Identify whether the following compounds are expected to be soluble or insoluble based on the solubility rules given in the Introduction. \( \mathrm{NH}_{4} \mathrm{NC} \) \[ \mathrm{BaCO}_{3} \] \[ \mathrm{AgOH} \] \[ \mathrm{Fe} 2\left(\mathrm{SO}_{4}\right)_{3} \] \[ \mathrm{NiCO}_{3} \] \[ \mathrm{CoCO}_{3} \] \[ \mathrm{CaCl}_{2} \] \[ \mathrm{A}_{\mathrm{b} C l} \] \[ \mathrm{NH}_{4} \mathrm{OH} \]
Expert Answer
From the above table we can conclude that : -> NH4NO3 is soluble in wat