Home /
Expert Answers /
Chemistry /
identify-the-missing-information-for-each-neutral-isotope-a-se-atom-has-a-mass-number-of-77-dete-pa308
(Solved): Identify the missing information for each neutral isotope. A Se atom has a mass number of 77 . Dete ...
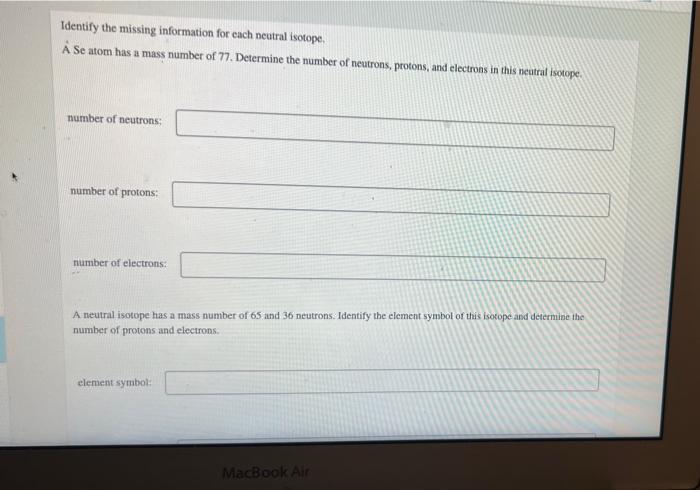
Identify the missing information for each neutral isotope. A Se atom has a mass number of 77 . Determine the number of neutrons, protons, and electrons in this nentral isotope. number of neutrons: number of protons: number of electrons: A neutral isotope has a mass number of 65 and 36 neutrons. Identify the element symbol of this isotope and determine the number of protons and electrons. clement symbol:
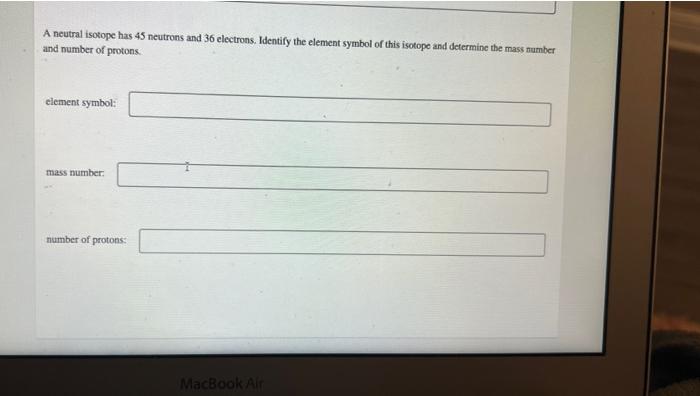
A neutral isotope has 45 neutrons and 36 electrons. Identify the element symbol of this isotope and determine the mass number and number of protons. element symbol: mass number: number of protons: