Home /
Expert Answers /
Chemistry /
identify-the-limiting-reactant-in-the-reaction-of-diphosphorus-pentoxide-and-water-to-form-mat-pa114
(Solved): Identify the limiting reactant in the reaction of diphosphorus pentoxide and water to form \( \mat ...
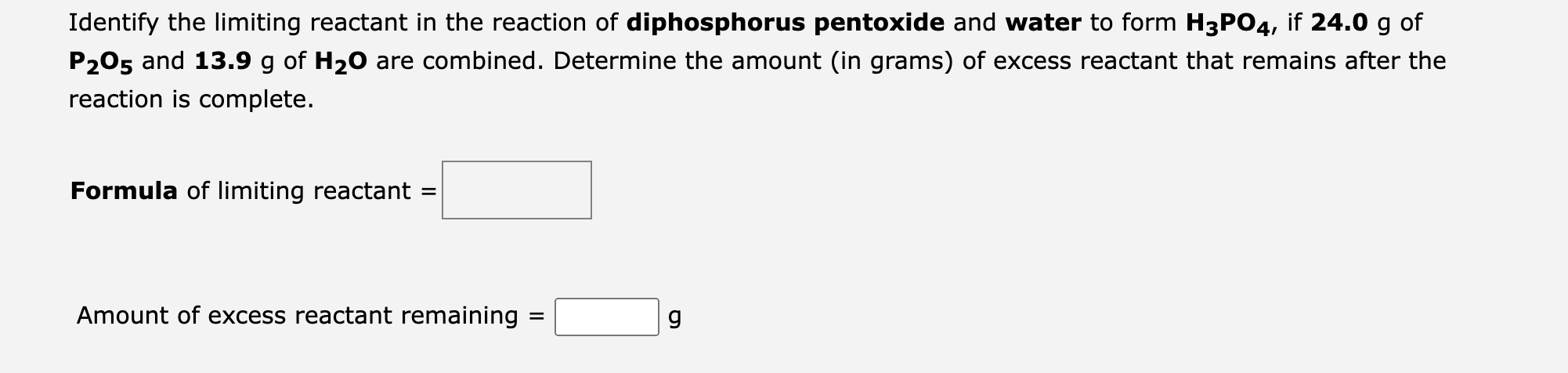
Identify the limiting reactant in the reaction of diphosphorus pentoxide and water to form \( \mathbf{H}_{3} \mathbf{P O}_{4} \), if \( \mathbf{2 4 . 0} \mathrm{g} \mathrm{of}^{\text {shan }} \) \( \mathbf{P}_{\mathbf{2}} \mathbf{O}_{\mathbf{5}} \) and \( \mathbf{1 3 . 9} \mathrm{g} \) of \( \mathbf{H}_{\mathbf{2}} \mathbf{O} \) are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete. Formula of limiting reactant \( = \) Amount of excess reactant remaining \( = \)
Consider the reaction of tin with potassium hydroxide and water. \[ \mathrm{Sn}(\mathrm{s})+2 \mathrm{KOH}(\mathrm{aq})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \longrightarrow \mathrm{K}_{2} \mathrm{Sn}(\mathrm{OH})_{6}(\mathrm{~s})+2 \mathrm{H}_{2}(\mathrm{~g}) \] Determine the limiting reactant in a mixture containing \( 199 \mathrm{~g} \) of \( \mathbf{S n}, 161 \mathrm{~g} \) of \( \mathbf{K O H} \), and \( 141 \mathrm{~g} \) of \( \mathbf{H}_{\mathbf{2}} \mathbf{O} \). Calculate the maximum mass (in grams) of potassium hydroxystannate, \( \mathbf{K}_{\mathbf{2}} \mathbf{S n}(\mathbf{O H})_{6} \), that can be produced in the reaction. The limiting reactant is: \[ \begin{array}{l} \mathrm{KOH} \\ \mathrm{H}_{2} \mathrm{O} \end{array} \] \( \mathrm{Sn} \) Amount of \( \mathbf{K}_{2} \mathbf{S n}(\mathbf{O H})_{6} \) formed \( = \) g