Home /
Expert Answers /
Biology /
ibuprofen-is-an-over-the-counter-drug-that-blocks-a-class-of-prostaglandins-that-cause-inflammation-pa939
(Solved): Ibuprofen is an over-the-counter drug that blocks a class of prostaglandins that cause inflammation ...
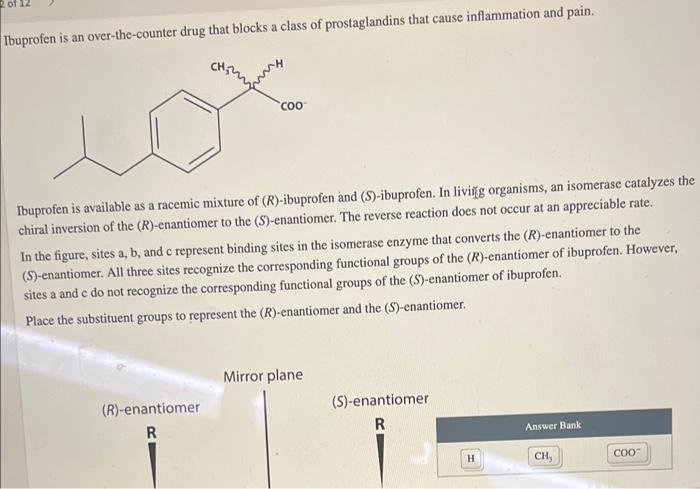
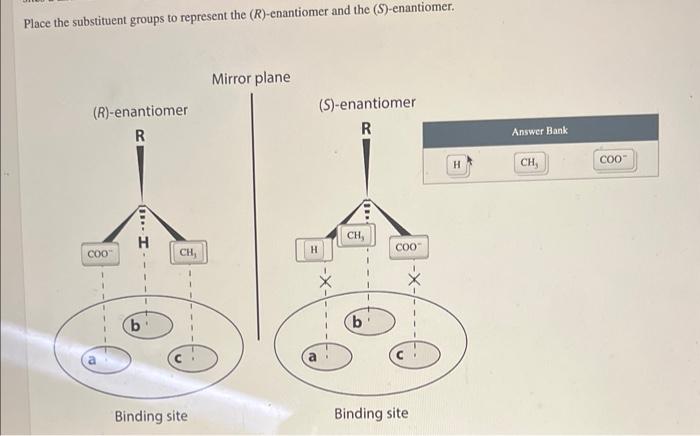

Ibuprofen is an over-the-counter drug that blocks a class of prostaglandins that cause inflammation and pain. Ibuprofen is available as a racemic mixture of \( (R) \)-ibuprofen and \( (S) \)-ibuprofen. In livily \( g \) organisms, an isomerase catalyzes the chiral inversion of the \( (R) \)-enantiomer to the \( (S) \)-enantiomer. The reverse reaction does not occur at an appreciable rate. In the figure, sites a, b, and c represent binding sites in the isomerase enzyme that converts the (R)-enantiomer to the (S)-enantiomer. All three sites recognize the corresponding functional groups of the \( (R) \)-enantiomer of ibuprofen. However, sites a and c do not recognize the corresponding functional groups of the (S)-enantiomer of ibuprofen. Place the substituent groups to represent the \( (R) \)-enantiomer and the \( (S) \)-enantiomer.
Place the substituent groups to represent the \( (R) \)-enantiomer and the (S)-enantiomer.
The (S)-enantiomer of ibuprofen is 100 times more efficacious for pain relief than is the ( \( R \) )-enantiomer. Drug companies sometimes make enantiomerically pure versions of drugs that were previously sold as racemic mixes, such as esomeprazole (Nexium) and escitalopram (Lexapro). Since (S)-ibuprofen is more effective, why do drug companies not sell enantiomerically pure (S)-ibuprofen? The presence of the \( (R) \)-enantiomer prevents chiral inversion, and inactivation, of the \( (S) \)-enantiomer. The conversion molecule binds the (S)-enantiomer and keeps it out of circulation. It is unnecessary because an enzyme converts the less effective enantiomer to the effective enantiomer. It is unnecessary because both enantiomers relieve pain at equal levels in a racemic mixture.
Expert Answer
Option A is correct. It is called pure when it has, within the limits