Home /
Expert Answers /
Chemistry /
i-need-help-with-this-lab-please-hydrolysis-and-buffers-objectives-1-to-use-the-mathrm-ph-pa531
(Solved): I Need Help With This Lab Please. Hydrolysis and Buffers Objectives: 1) To use the \( \mathrm{pH} \) ...
I Need Help With This Lab Please.













Hydrolysis and Buffers Objectives: 1) To use the \( \mathrm{pH} \) of solutions of the salts of weak acids and bases to determine the \( K_{\text {s }} \) of the weak acid or \( K_{b} \) of the weak base. 2) To observe and calculate the effect of the addition of a strong acid or base has on a buffer compared their effect on water. Background: Hydrolysis (See also hittps: /lyoutu be/Z.0094L.5dzik, and hitps://youtu belleANa.Yr5Ag) In the first part of this lab we will look at a series of solutions containing different ions and determine their pH's using a pH electrode. At the same time we will have to be ready to answer the question, "What ions affect the \( \mathrm{pH} \) of the solution?" In order to answer this question, we need to review a few things. By now we have leamed that a weak acid partially dissociates in solution. For our discussion here we will look at the weak acid HF. We can write an equilibrium expression showing the dissociation of \( \mathrm{HF} \) in aqueous solution. \[ \mathrm{HF}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{F}^{-} \quad \mathrm{K}_{\mathrm{a}}=\frac{\left.\left[\mathrm{H}_{3} \mathrm{O}^{+}\right] \mathrm{F}-\right]}{[\mathrm{HF}]} \] Since \( \mathrm{HF} \) is a weak acid, this means that \( \mathrm{F}^{-1} \) acts as a weak base. The rule of thumb is the weaker the acid, the stronger a base its conjugate base is and vise versa. If we have a solution of \( \mathrm{F}^{-1} \) on its own, it can act as a base towards water. This is known as a hydrolysis reaction and the result is that a solution of fluoride ion will normally be basic. \[ \mathrm{F}^{+}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{HF}+\mathrm{OH} \quad \quad K_{b}=\frac{\left[\mathrm{HFF}^{-}\left[\mathrm{OH}^{-1}\right]\right.}{\left[\mathrm{F}^{-}\right]} \] From this information, we can conclude that, if we have a solution of an anion whose conjugate acid is a weak acid, that solution will be basic. On the other hand, if the conjugate acid of the anion is a strong acid, it will have little or no effect on solution \( \mathrm{pH} \). Cations can also affect solution pH. To start with, it can be said that if a cation affects solution \( \mathrm{pH} \), it will make a solution more acidic. We will look at two ways that cations affect solution \( \mathrm{pH} \) here. Protonated amines as a class of cations can act as weak acids for the same reasons that we've seen with anions of weak acids. In this lab, we will study both ammonium ion \( \left(\mathrm{NH}_{4}^{+}\right) \)and methylamonium ion \( \left(\mathrm{CH}_{2} \mathrm{NH}_{3}^{+}\right) \). The simplest protonated amine is ammonium ion. Ammonium ion can form through the hydrolysis of ammonia \[ \mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{NH}_{4}^{+}+\mathrm{OH}^{-} \quad \mathrm{K}_{b}=\frac{\left[\mathrm{NH}_{2}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{NH}_{3}\right]} \] Since ammonia is a weak base, ammonium ion can act as a weak acid.
Hydrolysis and Buffers 96 \[ \mathrm{NH}_{4}^{+}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{NH}_{3} \mathrm{~K}_{\mathrm{a}}=\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{NH}_{3}\right]}{\left[\mathrm{NH}_{4}^{+}\right]}=\frac{\mathrm{K}_{\mathrm{w}}}{\mathrm{K}_{\mathrm{b}}} \] Another class of cations we will look at is those whose hydroxide salts are not very soluble in water. This category covers a large host of cations since there aren't very many cations whose hydroxide salts are water soluble. It could be said that these cations as a group have a strong affinity for hydroxide ions and can pull hydroxide from a water molecule. We will look at copper(II) ion as an example. \[ \mathrm{Cu}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{Cu}(\mathrm{OH})^{+}+\mathrm{H}_{3} \mathrm{O}^{+} \quad \mathrm{K}_{\mathrm{a}}=\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{Cu}(\mathrm{OH})^{+}\right]}{\left[\mathrm{Cu}^{2+}\right]} \] This picture simplifies what really happens with some cations, but it does help us to get a clear picture of why the presence of a cation may cause a solution to be acidic. If the hydroxide salt of a cation is water soluble, then it is safe to assume that the presence of that cation in solution will not affect solution \( \mathrm{pH} \). Buffers (See also hitps://youtu be/5fA AHFiOtlg and https://youtu be/IV80M600v X 4). A solution that contains both the conjugate acid and conjugate base of a conjugate acid/base pair is said to be a buffer solution. A buffer solution has the ability to absorb a substantial amount of acid or base without having its pH change large amounts. This can be useful for many applications. Our bodies utilize a buffer system in the blood stream, which enables us to survive being able to ingest acidic food without having blood \( \mathrm{pH} \) change drastically, which would be fatal. If we have a solution that contains both \( \mathrm{HF} \) and \( \mathrm{F}^{-1} \), we could determine the hydronium ion concentration using the following equation, which is a manipulated form of the \( \mathrm{K}_{\mathrm{a}} \) expression for HF. \[ \left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\mathrm{K}_{\mathrm{a}} \frac{[\mathrm{HP}]}{\left[\mathrm{F}^{-}\right]} \] Since \( \mathrm{pH} \) is defined as \( -\log \left[\mathrm{H}_{3} \mathrm{O}^{+}\right] \)and \( \mathrm{pK}_{\mathrm{a}} \) is defined as \( -\log \left(\mathrm{K}_{\mathrm{s}}\right) \), we can rewrite the above equation. \[ \mathrm{pH}=\mathrm{pK}_{\mathrm{a}}+\log \frac{\left[\mathrm{F}^{-}\right]}{[\mathrm{HF}]} \] This is known as the Henderson-Hasselbach equation and is only useful with buffer solutions. The generic form of the equation is: \[ \mathrm{pH}=\mathrm{pK}_{\mathrm{a}}+\log \frac{\text { [Base] }}{\text { [Acid] }} \]
There are times when it is easier to think about this equation in terms of ratios of amounts of conjugate acid and base. Since [Base] \( =\frac{\text { mmole Base }}{\mathrm{mL} \text { Solution }} \), and \( [ \) Acid] \( ]=\frac{\text { mmole Acid }}{\text { mL Solution' }} \), and in a buffer the Base and Acid are both in the same solution ( \( \mathrm{mL} \) solution is the same in both cases), we can rewrite the equation to be. \[ \mathrm{pH}=\mathrm{pK}_{\mathrm{a}}+\log \left(\frac{\mathrm{mmole} \mathrm{Base}}{\mathrm{mmole} \text { Acid }}\right) \text {. } \] This form of the Henderson-Hasselbalch equation can be useful when calculating the \( \mathrm{pH} \) of a buffer after acid or base has been added. Other Useful Calculations: \[ \begin{array}{l} \mathrm{pH}=-\log \left[\mathrm{H}_{3} \mathrm{O}^{+}\right], \quad \mathrm{pOH}=-\log \left[\mathrm{OH}^{-}\right], \quad \mathrm{pH}+\mathrm{pOH}=\mathrm{pK}_{\mathrm{w}}=14.00 \\ {\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=10^{-p H} M, \text { and } \quad\left[\mathrm{OH}^{-}\right]=10^{-p \mathrm{pHH}} M} \end{array} \] Special Considerations: Solutions of hydrochloric acid, acetic acid, and sodium hydroxide can all cause burns to the skin and eyes. If you get any of these solutions on any part of your body, wash the area with lots of water. Solutions of sodium fluoride are toxic and can be absorbed through the \( s k i n \). If you get any sodium fluoride solution on your skin, wash immediately with lots of water. The other solutions used in this lab can be irritating to the skin. Exercise care when using them. Your instructor will show you the proper use of \( \mathrm{pH} \) meters. Pay careful attention to the directions you receive from your instructor. The \( \mathrm{pH} \) electrode should be immersed in a solution unless it is being rinsed. DO NOT LEA VE THE pH ELECTRODE OUT OF A SOLUTION FOR MORE THAN 30 SECONDS.
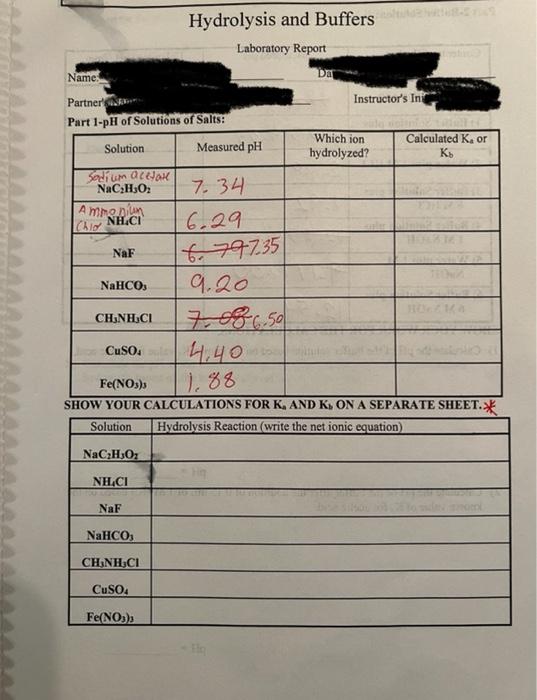
Hydrolysis and Buffers Laboratory Report Part 1-pH of Solutions of Salts:
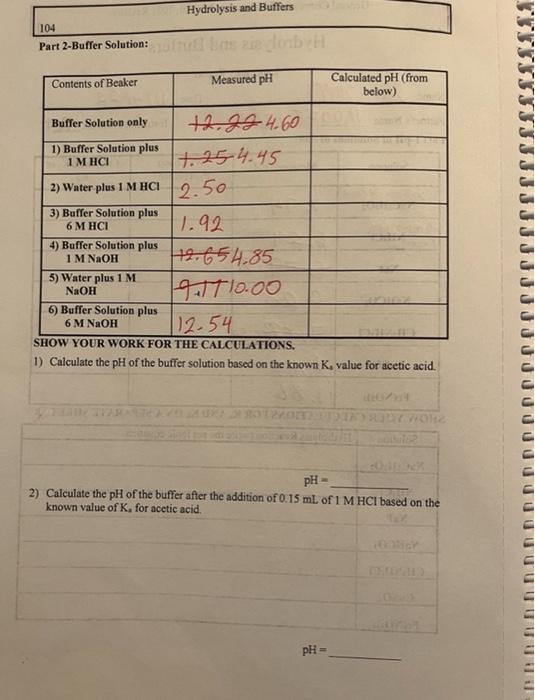
Part 2-Buffer Solution: 1) Calculate the \( \mathrm{pH} \) of the buffer solution based on the known \( \mathrm{K}_{4} \) value for acetic acid. 2) Calculate the pH of the buffer after the addition of \( 0.15 \mathrm{~mL} \) of \( 1 \mathrm{M} \mathrm{HCl} \) based on the known value of \( K_{,} \)for acetic acid.
General Chemistry II Laboratory Manual, 2021 Revision. 3) Calculate the \( \mathrm{pH} \) of \( 15 \mathrm{~mL} \) of water after \( 0.15 \mathrm{~mL} \) of \( 1 \mathrm{M} \mathrm{HCl} \) is added. \[ \mathrm{pH}= \] 4) Calculate the \( \mathrm{pH} \) of the buffer after the addition of \( 0.15 \mathrm{~mL} \) of \( 6 \mathrm{MHCl} \) based on the known value of \( \mathrm{K} \), for acetic acid. 5) Calculate the pH of the buffer solution after the addition of \( 0.15 \mathrm{~mL} \) of sodium hydroxide based on the known value of \( \mathrm{K}_{\mathrm{a}} \) for acetic acid.
6) Calculate the \( \mathrm{pH} \) of water after the addition of \( 0.15 \mathrm{~mL} \) of \( 1 \mathrm{M} \mathrm{NaOH} \). \[ \mathrm{pH}= \] 7) Calculate the \( \mathrm{pH} \) of the buffer after the addition of \( 0.15 \mathrm{~mL} \) of \( 6 \mathrm{M} \mathrm{NaOH} \) based on the known value of \( K \), for acetic acid.
Part 3: Make a Buffer \( \mathrm{pH} \) needed: Instructor' Volume of \( 0.100 \mathrm{M} \mathrm{NaOH} \) solution needed to make this buffer. Measured pH: Show your calculations below.
Expert Answer
Answer (3) pH = 2 Molarity of HCl = 1M Volume of HCl = 0.15 mL = 0.15 x 10-3 L Moles of H