Home /
Expert Answers /
Chemistry /
i-just-need-answers-for-this-4-question-please-help-a-typical-barometric-pressure-in-denver-colora-pa817
(Solved): I just need answers for this 4 question please help A typical barometric pressure in Denver, Colora ...
A typical barometric pressure in Denver, Colorado, is . What is this pressure in bar?
A medical laboratory catalog describes the pressure in a cylinder of a gas as . What is this pressure in psi? (1 psi = 6894.8
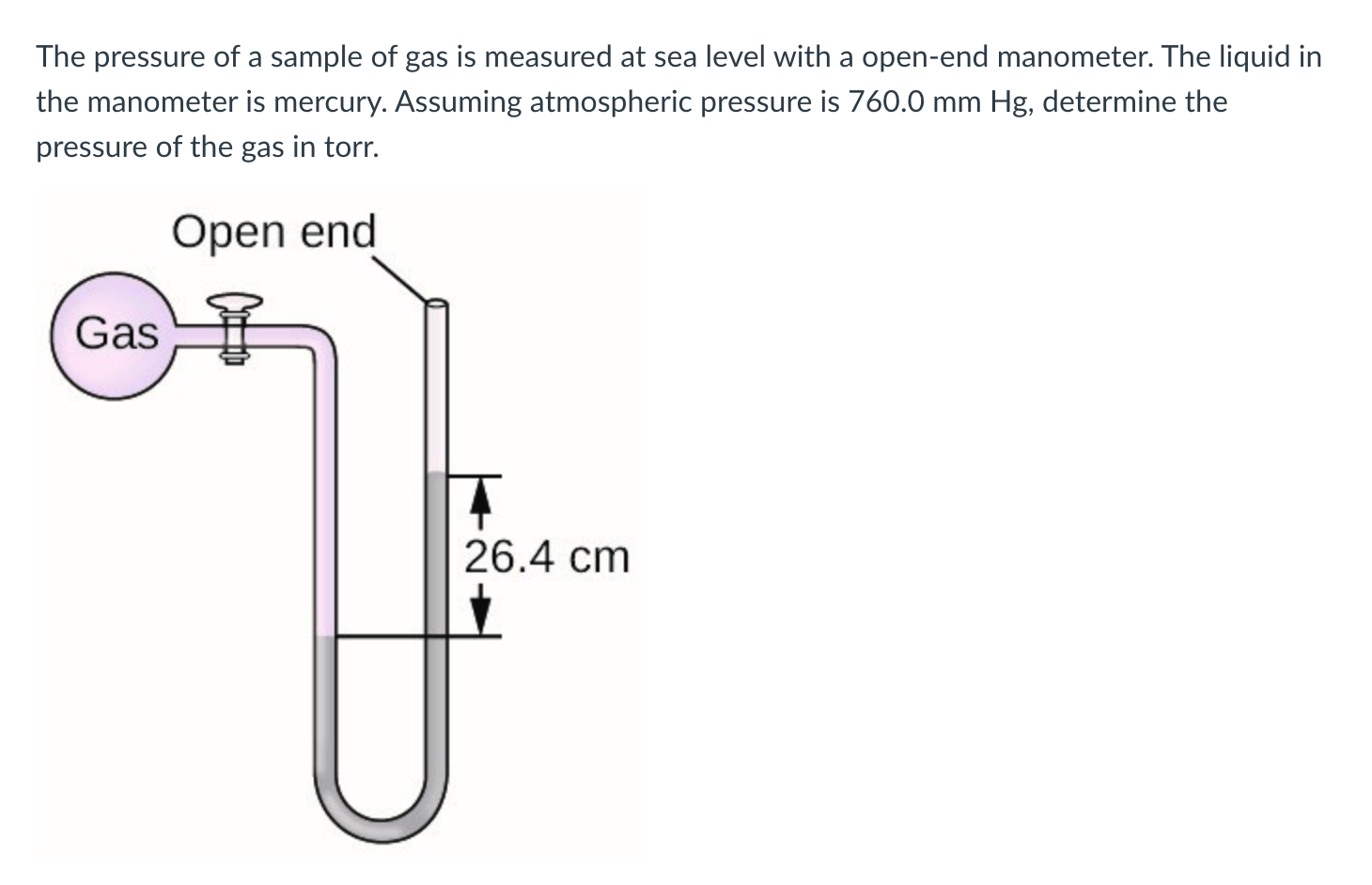
The pressure of a sample of gas is measured at sea level with a open-end manometer. The liquid in the manometer is mercury. Assuming atmospheric pressure is , determine the pressure of the gas in torr.
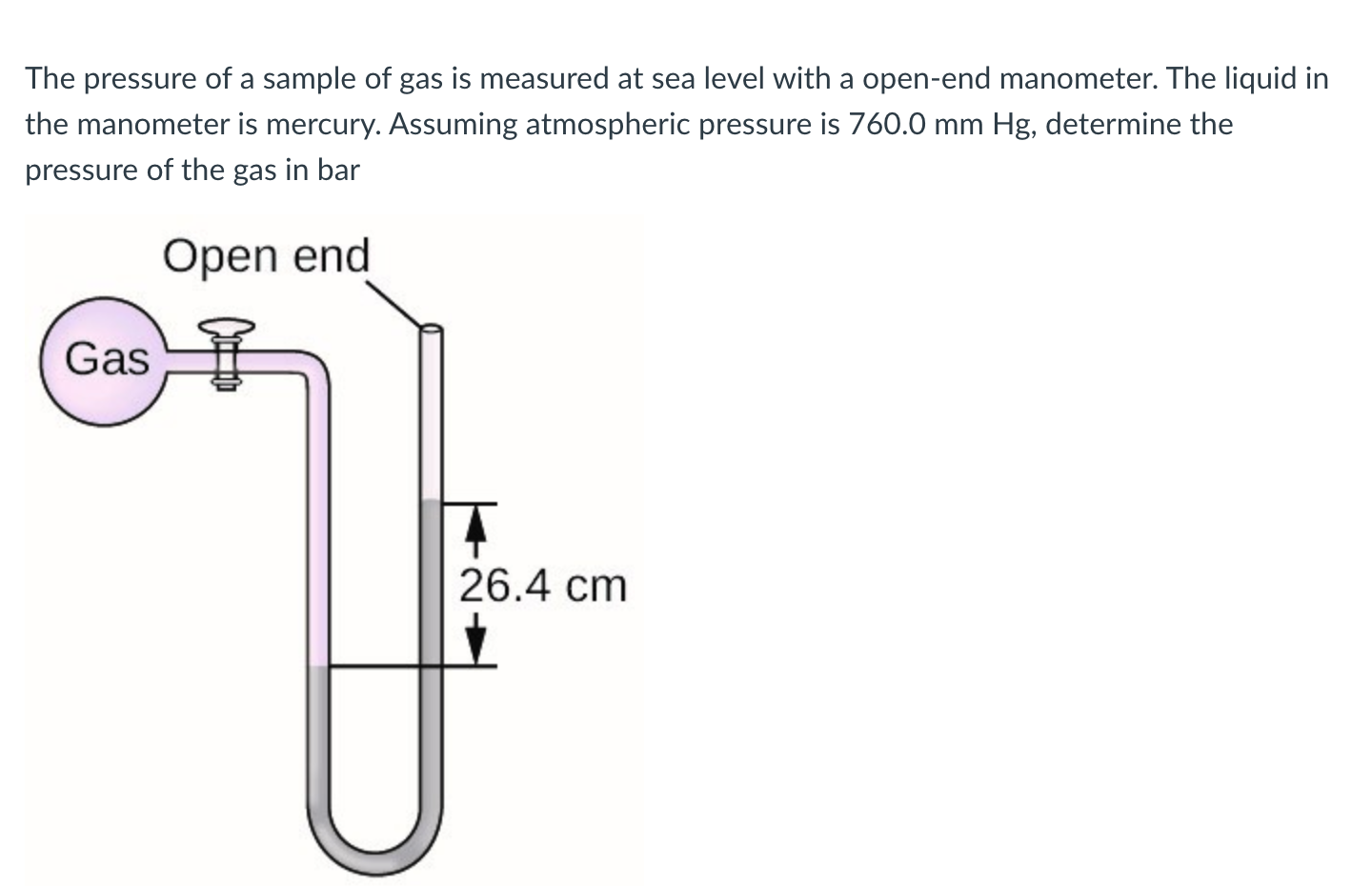
The pressure of a sample of gas is measured at sea level with a open-end manometer. The liquid in the manometer is mercury. Assuming atmospheric pressure is , determine the pressure of the gas in bar
Expert Answer
1 mmHg = 0.00133322 barto convert 615.5 mmHg to bar multiply by the conversion factor:615.5 mmHg × 0.00133322 bar/mmHg = 0.8204 bar