(Solved): hydrogen peroxide by the catalase in your body's cells. Catalase can be obtained from many sources, ...
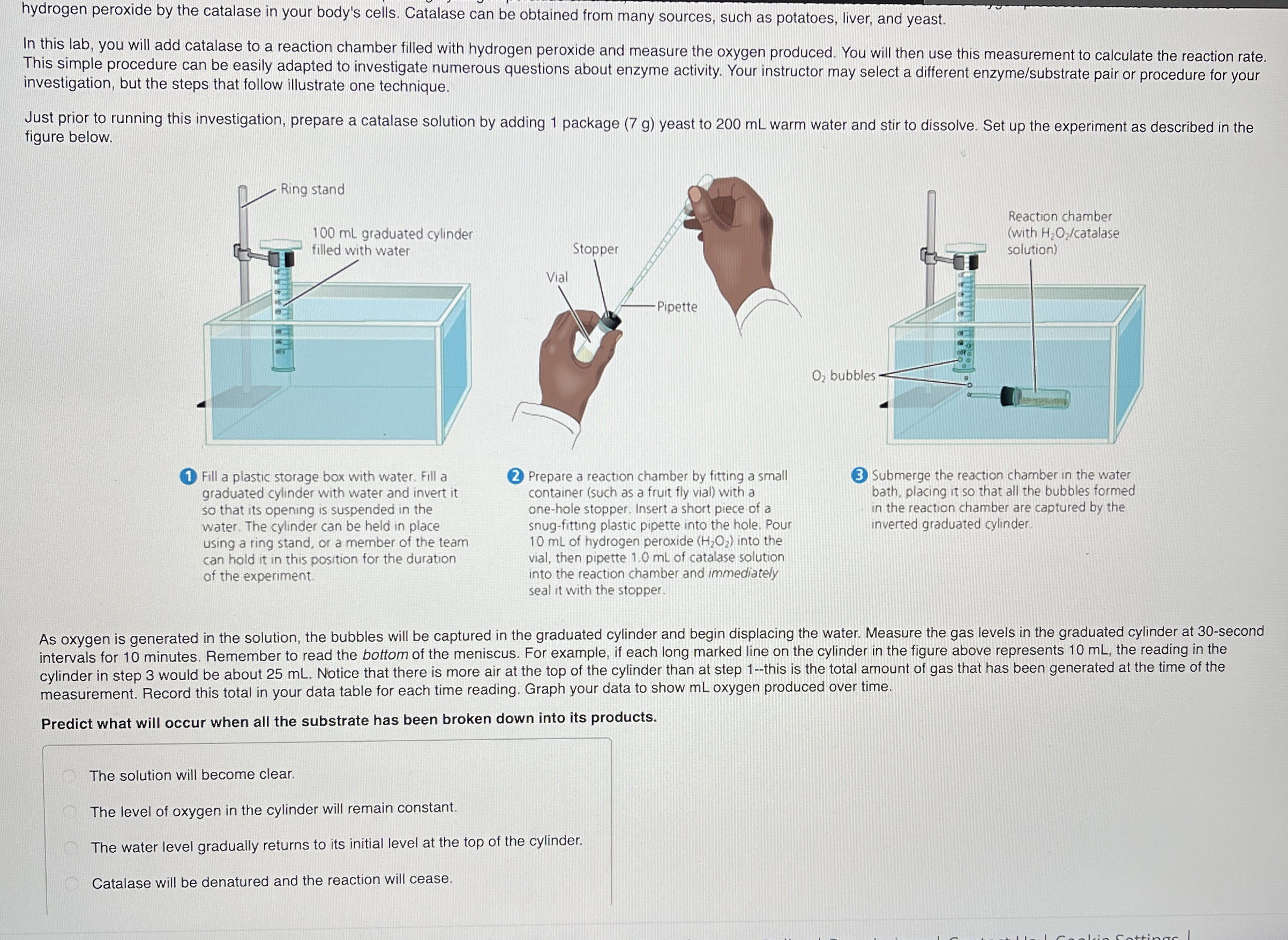
hydrogen peroxide by the catalase in your body's cells. Catalase can be obtained from many sources, such as potatoes, liver, and yeast. In this lab, you will add catalase to a reaction chamber filled with hydrogen peroxide and measure the oxygen produced. You will then use this measurement to calculate the reaction rate. This simple procedure can be easily adapted to investigate numerous questions about enzyme activity. Your instructor may select a different enzyme/substrate pair or procedure for your investigation, but the steps that follow illustrate one technique. Just prior to running this investigation, prepare a catalase solution by adding 1 package
(7g)yeast to 200 mL warm water and stir to dissolve. Set up the experiment as described in the figure below. (1) Fill a plastic storage box with water. Fill a graduated cylinder with water and invert it so that its opening is suspended in the water. The cylinder can be held in place using a ring stand, or a member of the team can hold it in this position for the duration of the experiment. (2) Prepare a reaction chamber by fitting a small container (such as a fruit fly vial) with a one-hole stopper. Insert a short piece of a snug-fitting plastic pipette into the hole. Pour 10 mL of hydrogen peroxide
(H_(2)O_(2))into the vial, then pipette 1.0 mL of catalase solution into the reaction chamber and immediately seal it with the stopper. Submerge the reaction chamber in the water bath, placing it so that all the bubbles formed in the reaction chamber are captured by the inverted graduated cylinder. As oxygen is generated in the solution, the bubbles will be captured in the graduated cylinder and begin displacing the water. Measure the gas levels in the graduated cylinder at 30 -second intervals for 10 minutes. Remember to read the bottom of the meniscus. For example, if each long marked line on the cylinder in the figure above represents 10 mL , the reading in the cylinder in step 3 would be about 25 mL . Notice that there is more air at the top of the cylinder than at step 1-this is the total amount of gas that has been generated at the time of the measurement. Record this total in your data table for each time reading. Graph your data to show mL oxygen produced over time. Predict what will occur when all the substrate has been broken down into its products. The solution will become clear. The level of oxygen in the cylinder will remain constant. The water level gradually returns to its initial level at the top of the cylinder. Catalase will be denatured and the reaction will cease.hydrogen peroxide by the catalase in your body's cells. Catalase can be obtained from many sources, such as potatoes, liver, and yeast. In this lab, you will add catalase to a reaction chamber filled with hydrogen peroxide and measure the oxygen produced. You will then use this measurement to calculate the reaction rate. This simple procedure can be easily adapted to investigate numerous questions about enzyme activity. Your instructor may select a different enzyme/substrate pair or procedure for your investigation, but the steps that follow illustrate one technique. Just prior to running this investigation, prepare a catalase solution by adding 1 package
(7g)yeast to 200 mL warm water and stir to dissolve. Set up the experiment as described in the figure below. (1) Fill a plastic storage box with water. Fill a graduated cylinder with water and invert it so that its opening is suspended in the water. The cylinder can be held in place using a ring stand, or a member of the team can hold it in this position for the duration of the experiment. (2) Prepare a reaction chamber by fitting a small container (such as a fruit fly vial) with a one-hole stopper. Insert a short piece of a snug-fitting plastic pipette into the hole. Pour 10 mL of hydrogen peroxide
(H_(2)O_(2))into the vial, then pipette 1.0 mL of catalase solution into the reaction chamber and immediately seal it with the stopper. Submerge the reaction chamber in the water bath, placing it so that all the bubbles formed in the reaction chamber are captured by the inverted graduated cylinder. As oxygen is generated in the solution, the bubbles will be captured in the graduated cylinder and begin displacing the water. Measure the gas levels in the graduated cylinder at 30 -second intervals for 10 minutes. Remember to read the bottom of the meniscus. For example, if each long marked line on the cylinder in the figure above represents 10 mL , the reading in the cylinder in step 3 would be about 25 mL . Notice that there is more air at the top of the cylinder than at step 1-this is the total amount of gas that has been generated at the time of the measurement. Record this total in your data table for each time reading. Graph your data to show mL oxygen produced over time. Predict what will occur when all the