Home /
Expert Answers /
Chemistry /
hydrogen-gas-burns-in-an-atmosphere-of-bromine-gas-to-produce-hydrogen-bromide-gas-according-to-the-pa653
(Solved): Hydrogen gas burns in an atmosphere of bromine gas to produce hydrogen bromide gas according to the ...
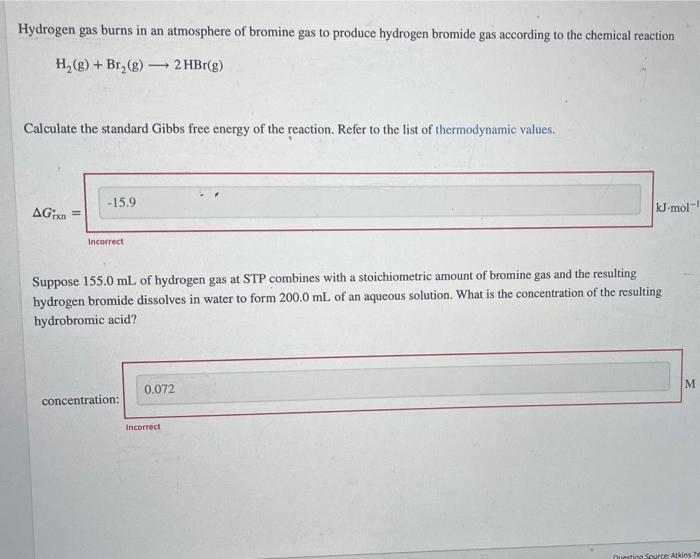
Hydrogen gas burns in an atmosphere of bromine gas to produce hydrogen bromide gas according to the chemical reaction H?(g) + Br?(g) - - 2 HBr(g) Calculate the standard Gibbs free energy of the reaction. Refer to the list of thermodynamic values. -15.9 AGTxn kJ-mol-1 Incorrect Suppose 155.0 mL of hydrogen gas at STP combines with a stoichiometric amount of bromine gas and the resulting hydrogen bromide dissolves in water to form 200.0 mL of an aqueous solution. What is the concentration of the resulting hydrobromic acid? 0.072 M concentration: Questing Source: Atkins Te Incorrect