Home /
Expert Answers /
Chemistry /
how-would-i-create-a-balanced-equation-and-what-is-the-theoretical-yield-nbsp-introduction-the-oxi-pa345
(Solved): how would i create a balanced equation and what is the theoretical yield? Introduction The oxi ...
how would i create a balanced equation and what is the theoretical yield?
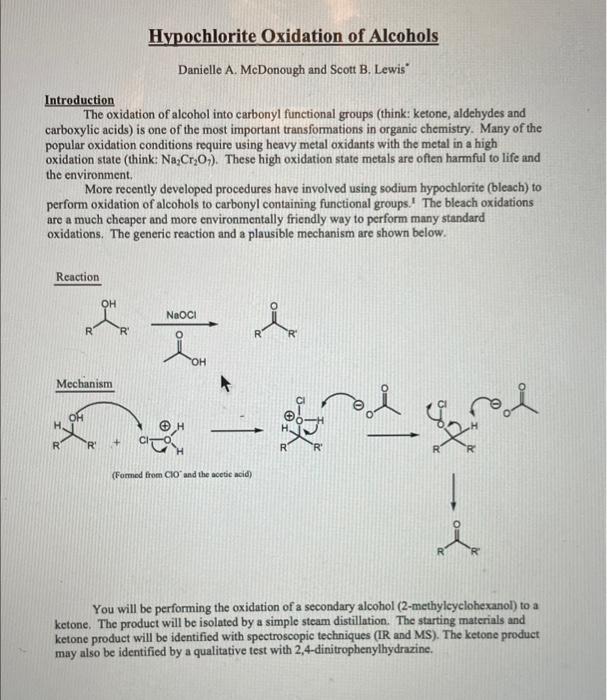





Introduction The oxidation of alcohol into carbonyl functional groups (think: ketone, aldehydes and carboxylic acids) is one of the most important transformations in organic chemistry. Many of the popular oxidation conditions require using heavy metal oxidants with the metal in a high oxidation state (think: \( \mathrm{Na}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7} \) ). These high oxidation state metals are often harmful to life and the environment, More recently developed procedures have involved using sodium hypochlorite (bleach) to perform oxidation of alcohols to carbonyl containing functional groups.' The bleach oxidations are a much cheaper and more environmentally friendly way to perform many standard oxidations. The generic reaction and a plausible mechanism are shown below. Reaction Mechanism (Forned from \( \mathrm{ClO}^{\circ} \) and the acetic acid) You will be performing the oxidation of a secondary alcohol (2-methylcyclohexanol) to a ketone. The product will be isolated by a simple steam distillation. The starting materials and ketone product will be identified with spectroscopic techniques (IR and MS). The ketone product may also be identified by a qualitative test with 2,4-dinitrophenylhydrazine.
Pre-Lab Be sure your pre-lab report includes all the things (and is written in the style) that your instructor normally requires. But at minimum, it should include: Title Purpose A data table of physical propertics and amounts/volumes of reagents The reaction equation shown using 2-methylcyclohexanol Calculation of the theoretical yield of product ketone The mechanism using 2-methylcyclohexanol Flow chart or procedure In addition, answer the following question (after the flow chart): Why are tertiary alcohols resistant to oxidation? Note: depending on the time of the semester, you may have to look this up on your own. Rrocedure To a clean \( 100 \mathrm{~mL} \) round bottom flask, add \( 3.0 \mathrm{~mL} \) of 2 -methylcyclohexanol and a stir bar. Clamp your flask such that it is half submerged in a Crystallization Dish of water which is sitting on a stirring hot plate. Turn the hot plate on. (The proper setting should be written an each hot plate in sharpic on the right side of the hot plate. Generally betheen 2 and 3 will get you to the proper femperature (between \( 40-47^{\circ} \mathrm{C} \) ].) In a beaker, mix \( 35.0 \mathrm{~mL} \) of household bleach and \( 3.0 \mathrm{~mL} \) of glacial acetic acid. After swirling the mixture for one minute (in the hood), add it to a separatory funnel that is hung such that the tip of the funnel is over or just inside of your round bottom flask. Note: Do not allow the separatory funnel to completely "fill/seal" the opening to the round bottom flask. Carefully open the stopcock on the separatory funnel to allow for a slow drip of the bleach/acetic acid mixture. The indition should be at such a pace as to take about 15 minutes for the entire addition. Once the addition is complete, check to see if a faint yellow color is present (a piece of white paper behind the flask may help). If the faint yellow color is not present, ask your instructor if you should add more bleach. If more bleach is added, the reaction mixture should be stirred for an additional 15 minutes. At the end of the stir time, add \( 3.0 \mathrm{~mL} \) of a \( 10 \% \mathrm{NaHSO}_{3} \) (sodium bisulfite) and be sure no faint yellow color is present. Check the \( \mathrm{pH} \) of the solution with pH paper. If you want to confirm your pH readings from the paper, ask for a check with one of the \( \mathrm{pH} \) meters. If it is below 7, add \( 6.0 \mathrm{~mL} \) of \( 6 \mathrm{M} \mathrm{NaOH} \) while continuing to stir. Check the \( \mathrm{pH} \) again. If the \( \mathrm{pH} \) is still below 7 , continue adding \( 6 \mathrm{M} \mathrm{NaOH} \) drop-wise with continual monitoring of the \( \mathrm{pH} \). The \( \mathrm{pH} \) should not go above 8 during these additions, so be careful with how much \( \mathrm{NaOH} \) in total gets added. Set up a simple distillation apparatus by attaching a 3-way connector and a condenser to the round bottom flask. With a gentle flow of water through the condenser place the round bottom flask in a heating mantle and distill out 7 to \( 10 \mathrm{~mL} \) total volume of liquids. It is very important that the temperature of the distillation does not go above \( 100^{\circ} \mathrm{C} \). If this happens, turn the heat off and remove the heat source immediately!
Place the liquid into a clean separatory funnel and drain out the water layer. Pour out the organic layer into a small Erlenmeyer flask and dry it with \( \mathrm{CaCl}_{2} \). Decant the dry liquid into a clean, dry pre-weighed Erlenmeyer and weigh the product. Optional Once weighed, take out 3 drops of the product and place in a small vial. Add \( 1.0 \mathrm{~mL} \) of \( 95 \% \) ethanol and 10 drops of the 2,4-DNP reagent. Shake (and gently warm if necessary) until solids form. Gravity filter this solid material and wash twice with small portions \( (1-2 \mathrm{~mL}) \) of \( 95 \% \) ethanol. Allow the solid time to dry (1-2 days) and then obtain and record its melting point. Note: The DNP reagent would give no product if reacted with the starting alcohol. Explain in your report why you would experf this difference in reactivity. Post-Lab Be sure your post-lab report includes all the things (and is written in the style) that your instructor normally requires. But at minimum, it should include: Percent yield of ketone product obtained Melting point of the 2,4-dinitrophenylhydrozone made from the product ketone reaction with 2,4-dinitrophenylhydrazine \( (2,4-\mathrm{DNP}) \) (lit. \( \mathrm{mp}=137^{\circ} \mathrm{C} \) ). Identification and discussion of spectra for both the starting alcohol and the product ketone Be sure to turn in copies of the spectra with your final report 1. Mohrig, J. R.; Nienhuis, D. M.; Linck, C. F.; Van Zoeren, C.; Fox, B. G.; Mahaffy, P.G. J. Chem. Ed. 1985, 62 (6), 519.