Home /
Expert Answers /
Chemistry /
how-many-lone-pairs-of-electrons-are-found-on-the-indicated-atom-one-two-three-four-none-for-the-pa869
(Solved): How many lone pairs of electrons are found on the indicated atom? one two three four none For the ...

How many lone pairs of electrons are found on the indicated atom? one two three four none
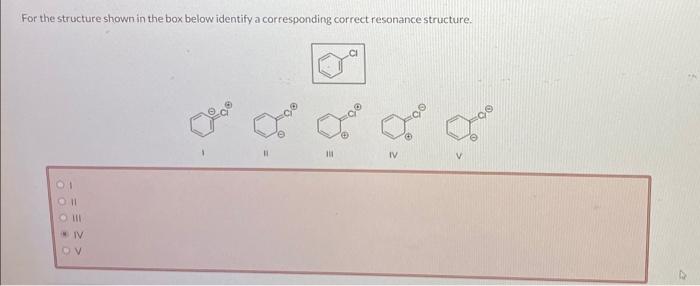
For the structure shown in the box below identify a corresponding correct resonance structure.
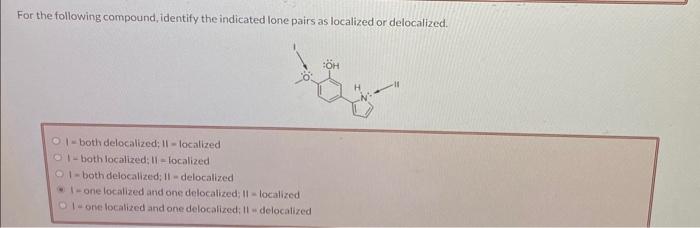
For the following compound, identify the indicated lone pairs as localized or delocalized. I - both delocalized; 11 = localized 1- both localized: 11 = localized 1 - both delocalized; II - delocalized I - one localized and one delocalized; 11 - localized 1- one localized and one delocalized: II = delocalized
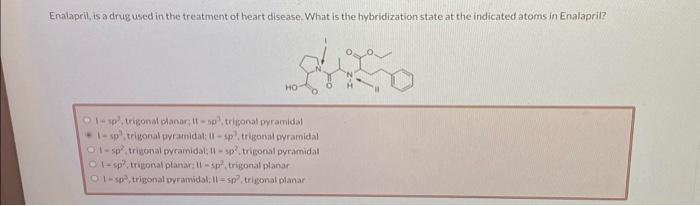
Enalaprit, is a drug used in the treatment of heart disease. What is the hybridization state at the indicated atoms in Enalapril? , trigonal planar; , trigonal pyramidal Q , trigonal pyramidal: , trigonal pyramidal . trigonal pyramidal: , trigonal pyramidal - sp², triegonal planar , trigonal planar -sp?
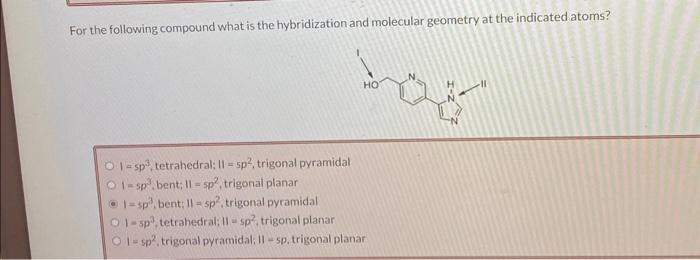
For the following compound what is the hybridization and molecular geometry at the indicated atoms? , tetrahedral: , trigonal pyramidal , bent; , trigonal planar , bent: , trigonal pyramidal , tetrahedral: , trigonal planar I , trigonal pyramidali II - sp, trigonal planar
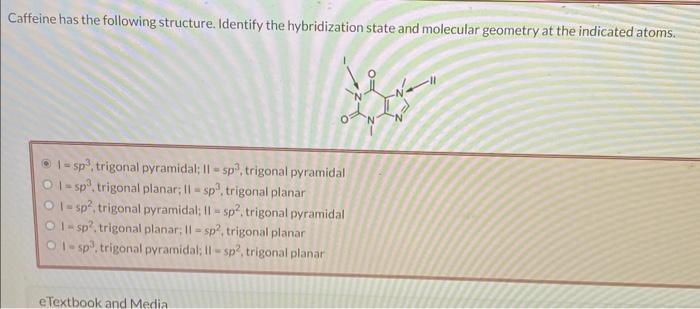
Caffeine has the following structure. Identify the hybridization state and molecular geometry at the indicated atoms. , trigonal pyramidal; , trigonal pyramidal , trigonal planar; , trigonal planar , trigonal pyramidal: , trigonal pyramidal 1 - , trigonal planar; , trigonal planar , trigonal pyramidal; II , trigonal planar
Expert Answer
According to Chegg's answering rule I am going to be answered three questions.1st Question:The Oxynium ion (oxygen atom with positive charge) is sp3 h