Home /
Expert Answers /
Chemistry /
how-many-grams-of-fe2o3-can-form-from-20-9g-of-o2-nbsp-1-show-the-strategy-for-solving-this-problem-pa985
(Solved): how many grams of Fe2O3 can form from 20.9g of O2? 1.show the strategy for solving this problem ...
how many grams of Fe2O3 can form from 20.9g of O2?
2. show the conversions required to solve this problem and calculate the grams of Fe2O3
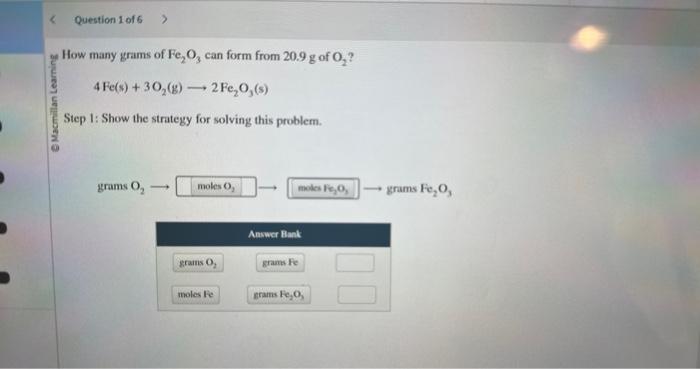

How many grams of \( \mathrm{Fe}_{2} \mathrm{O}_{3} \) can form from \( 20.9 \mathrm{~g} \mathrm{of} \mathrm{O}_{2} \) ? \[ 4 \mathrm{Fe}(\mathrm{s})+3 \mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{Fe}_{2} \mathrm{O}_{3}(\mathrm{~s}) \] Step 1: Show the strategy for solving this problem.
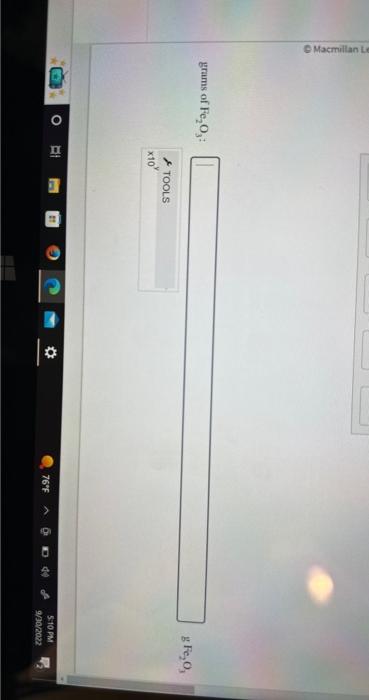
Step 2: Show the conversions required to solve this problem and calculate the grams of \( \mathrm{Fe}_{2} \mathrm{O}_{3} \).
grams of \( \mathrm{Fe}_{2} \mathrm{O}_{3} \) :