Home /
Expert Answers /
Chemistry /
how-do-you-do-1-19-how-do-we-know-which-h-can-or-cannot-be-replaced-with-a-br-nbsp-18-bromoaceton-pa333
(Solved): how do you do 1.19. how do we know which H can or cannot be replaced with a Br. 18 Bromoaceton ...
how do you do 1.19. how do we know which H can or cannot be replaced with a Br.
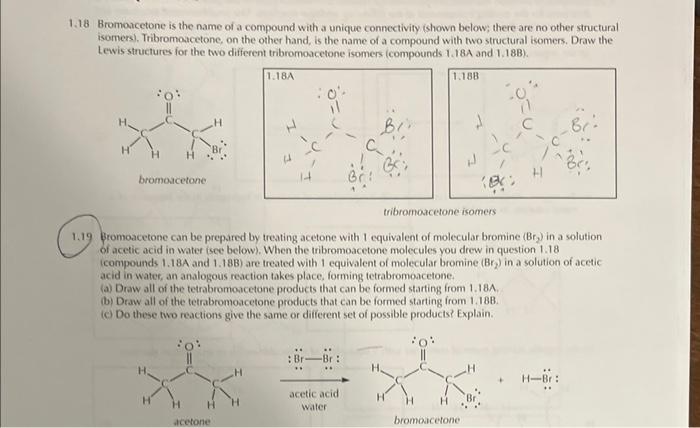
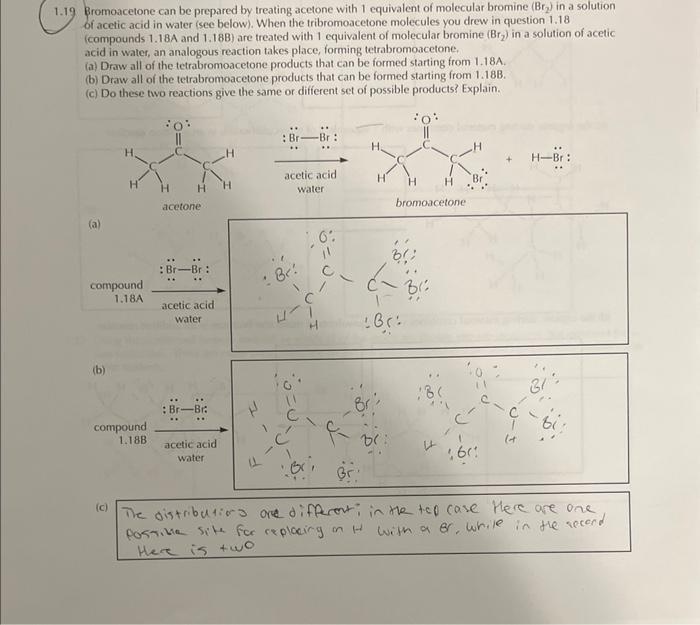


18 Bromoacetone is the name of a compound with a unique connectivity (shown below; there are no other structural isomers). Tribromoacetone, on the other hand, is the name of a compound with two structural isomers. Draw the Lewis structures for the two different tribromoacetone isomers (compounds \( 1.18 \mathrm{~A} \) and \( 1.18 \mathrm{~B} \) ). troromoserone isomers \( 1.19 \) Bromoacetone can be prepared by treating acetone with 1 equivalent of molecular bromine \( \left(B r_{2}\right) \) in a solution of acetic acid in water isee below). When the tribromoacetone molecules you drew in question \( 1.18 \) (compounds \( 1.18 \mathrm{~A} \) and \( 1.18 \mathrm{~B}) \) are treated with 1 equivalent of molecular bromine \( \left(\mathrm{Br} \mathrm{r}_{2}\right. \) ) in a solution of acetic acid in water, an analogous reaction takes place, forming tetrabromoacetone. (a) Draw all of the tetrabromoacetone products that can be formed starting from 1.18A. (b) Draw all of the tetrabromoacetone products that can be formed starting ifom 1.188. (c) Do these two reactions give the same or different set of possible products? Explain.
9 Bromoacetone can be prepared by treating acetone with 1 equivalent of molecular bromine \( \left(\mathrm{Br}_{2}\right) \) in a solution of acetic acid in water (see below). When the tribromoacetone molecules you drew in question \( 1.18 \) (compounds \( 1.18 \mathrm{~A} \) and \( 1.18 \mathrm{~B} \) ) are treated with 1 equivalent of molecular bromine \( \left(\mathrm{Br}_{2}\right) \) in a solution of acetic acid in water, an analogous reaction takes place, forming tetrabromoacetone,