Home /
Expert Answers /
Advanced Physics /
help-nbsp-determine-the-cell-notation-for-the-redox-reaction-given-below-3-mathrm-cl-2-m-pa254
(Solved): help ! Determine the cell notation for the redox reaction given below. \( 3 \mathrm{Cl}_{2}(\m ...
help !



Determine the cell notation for the redox reaction given below. \( 3 \mathrm{Cl}_{2}(\mathrm{~g})+2 \mathrm{Fe}(\mathrm{s}) \rightarrow 6 \mathrm{Cl}^{-}(\mathrm{aq})+2 \mathrm{Fe}^{3+}(\mathrm{aq}) \) \( \mathrm{Cl}_{2}(\mathrm{~g}) \mid \mathrm{Cl}^{-}(\mathrm{aq}), \mathrm{Pt} \| \mathrm{Fe}(\mathrm{s}), \mathrm{Fe}^{3+}(\mathrm{aq}) \) \( \mathrm{Cl}^{-}(\mathrm{aq}), \mathrm{Cl}_{2}(\mathrm{~g})\left|\mathrm{Pt} \| \mathrm{Fe}^{3+}(\mathrm{aq})\right| \mathrm{Fe}(\mathrm{s}) \) \( \mathrm{Fe}(\mathrm{s}), \mathrm{Cl}_{2}(\mathrm{~g}) \| \mathrm{Fe}^{3+}(\mathrm{aq})\left|\mathrm{Cl}^{-}(\mathrm{aq})\right| \mathrm{Pt} \) \( \left.\mathrm{Fe}(\mathrm{s}) \mid \mathrm{Fe}^{3+}{ }^{-} \mathrm{aq}\right) \Vdash \mathrm{Cl}_{2}(\mathrm{~g}) \mid \mathrm{Cl}^{-}(\mathrm{aq}) ? \mathrm{Pt} \)
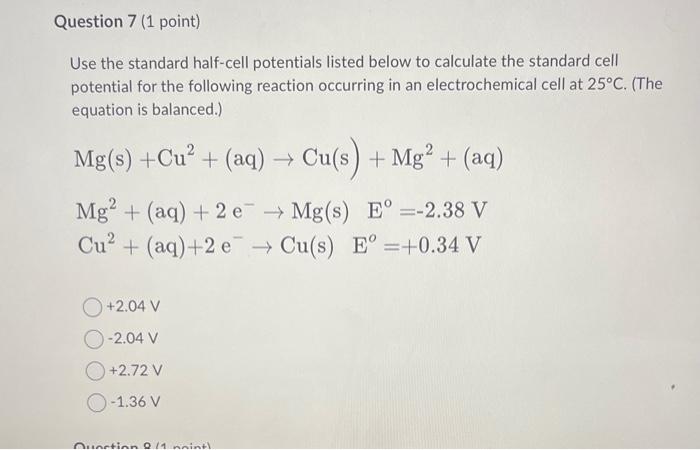
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at \( 25^{\circ} \mathrm{C} \). (The equation is balanced.) \[ \begin{array}{l} \mathrm{Mg}(\mathrm{s})+\mathrm{Cu}^{2}+(\mathrm{aq}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{Mg}^{2}+(\mathrm{aq}) \\ \mathrm{Mg} \\ \left.\mathrm{Cu}^{2}+(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s}) \mathrm{E}^{0}=-2.38 \mathrm{~V}\right) \quad \mathrm{E}^{0}=+0.34 \mathrm{~V} \\ \quad+2.04 \mathrm{~V} \\ -2.04 \mathrm{~V} \\ +2.72 \mathrm{~V} \\ -1.36 \mathrm{~V} \end{array} \]
Expert Answer
a) The cell notation for th