Home /
Expert Answers /
Chemistry /
given-the-following-thermochemical-equations-determine-the-molar-enthalpy-for-the-ionization-of-ac-pa166
(Solved): Given the following thermochemical equations, determine the molar enthalpy for the ionization of ac ...
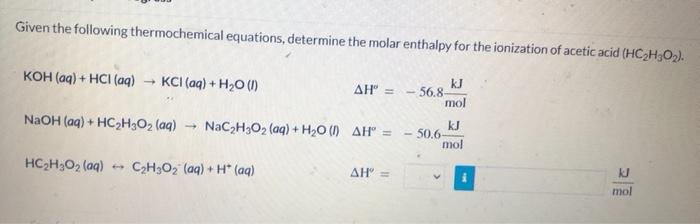
Given the following thermochemical equations, determine the molar enthalpy for the ionization of acetic acid \( \left(\mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}\right) \). \[ \begin{array}{ll} \mathrm{KOH}(a q)+\mathrm{HCl}(a q) \rightarrow \mathrm{KCl}(a q)+\mathrm{H}_{2} \mathrm{O}(l) & \Delta \mathrm{H}^{\circ}=-56.8 \frac{\mathrm{kJ}}{\mathrm{mol}} \\ \mathrm{NaOH}(a q)+\mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \rightarrow \mathrm{NaC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l) & \Delta \mathrm{H}^{\circ}=-50.6 \frac{\mathrm{kJ}}{\mathrm{mol}} \\ \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \leftrightarrow \mathrm{C}_{2} \mathrm{H}_{3} \mathrm{O}_{2}^{-}(a q)+\mathrm{H}^{*}(a q) & \Delta \mathrm{H}^{\circ}= \end{array} \]