Home /
Expert Answers /
Physics /
given-that-the-hf-molecule-rotates-about-an-axis-passing-through-its-center-find-the-bond-length-f-pa366
(Solved): Given that the HF molecule rotates about an axis passing through its center, find the bond length f ...
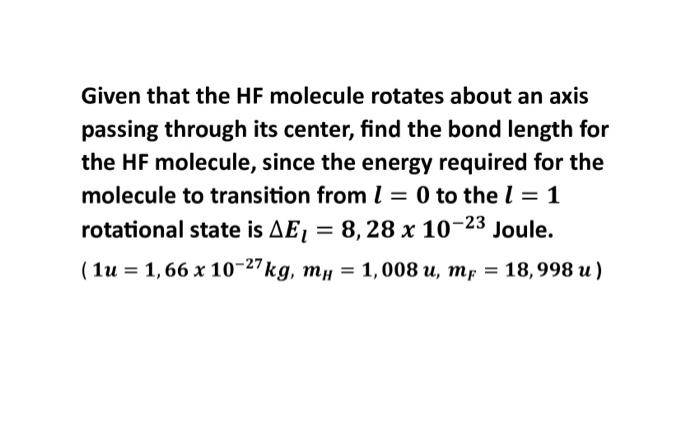
Given that the HF molecule rotates about an axis passing through its center, find the bond length for the HF molecule, since the energy required for the molecule to transition from to the rotational state is Joule.
Expert Answer
To find the bond length of the HF molecule, we can use the rotational energy formula: where E is the rotational energy, l is the rotational quantum number, ? is the reduced Planck's constant (1.0545718 × 10^(-34) J·s), and I is the moment of inertia of the molecule.Given that the energy required for the molecule to transition from l=0 to l=1 is del E = 8.28 × 10^23 J, we can equate this energy difference to the difference in rotational energy levels.