Home /
Expert Answers /
Chemical Engineering /
full-steps-and-solutions-please-a-for-an-iron-carbon-alloy-that-contains-2-pa782
(Solved): Full steps and solutions please a) For an iron-carbon alloy that contains \( 2 ...
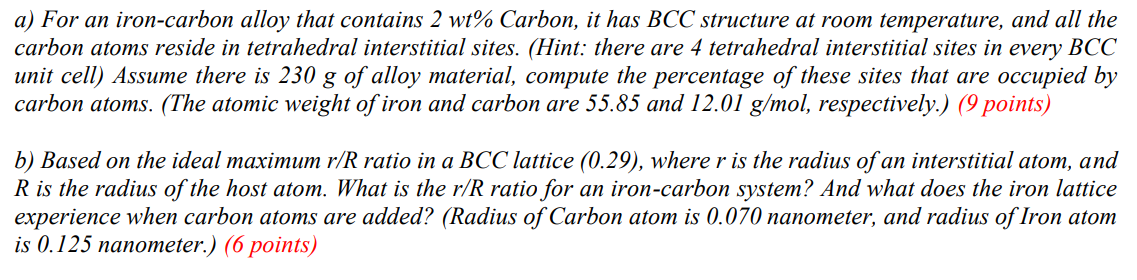
a) For an iron-carbon alloy that contains \( 2 w t \% \) Carbon, it has BCC structure at room temperature, and all the carbon atoms reside in tetrahedral interstitial sites. (Hint: there are 4 tetrahedral interstitial sites in every BCC unit cell) Assume there is \( 230 \mathrm{~g} \) of alloy material, compute the percentage of these sites that are occupied by carbon atoms. (The atomic weight of iron and carbon are \( 55.85 \) and \( 12.01 \mathrm{~g} / \mathrm{mol} \), respectively.) (9 points) b) Based on the ideal maximum \( r / R \) ratio in a BCC lattice (0.29), where \( r \) is the radius of an interstitial atom, and \( R \) is the radius of the host atom. What is the \( r / R \) ratio for an iron-carbon system? And what does the iron lattice experience when carbon atoms are added? (Radius of Carbon atom is \( 0.070 \) nanometer, and radius of Iron atom is \( 0.125 \) nanometer.) (6 points)